Inorganic chemistry q Calculating the standard emf of


- Slides: 8

Inorganic chemistry q Calculating the standard (emf) of an electrochemical cell. q Spontaneity of (Redox) reactions. Assiastance Lecturer Amjad Ahmed Jumaa www. soran. edu. iq 1

Calculating the standard (emf) of an electrochemical cell: In an electrochemical cell, electrons flow from one electrode to the other. This indicates that there is a voltage difference between the two electrodes. This voltage difference is called the electromotive force, or (emf) (E). The electromotive force is also called the cell voltage or cell potential, it is usually measured in volts. The electrode at which reduction occurs is called the cathode. The electrode at which oxidation occurs is called the anode. www. soran. edu. iq

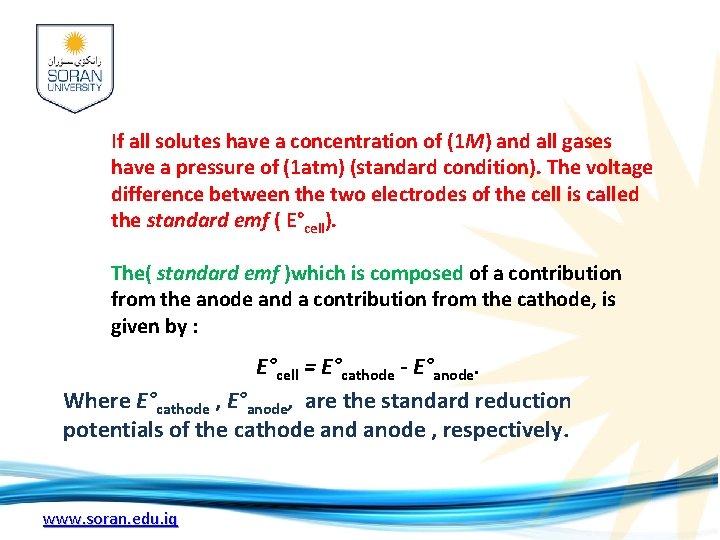
If all solutes have a concentration of (1 M) and all gases have a pressure of (1 atm) (standard condition). The voltage difference between the two electrodes of the cell is called the standard emf ( E°cell). The( standard emf )which is composed of a contribution from the anode and a contribution from the cathode, is given by : E°cell = E°cathode - E°anode. Where E°cathode , E°anode, are the standard reduction potentials of the cathode and anode , respectively. www. soran. edu. iq

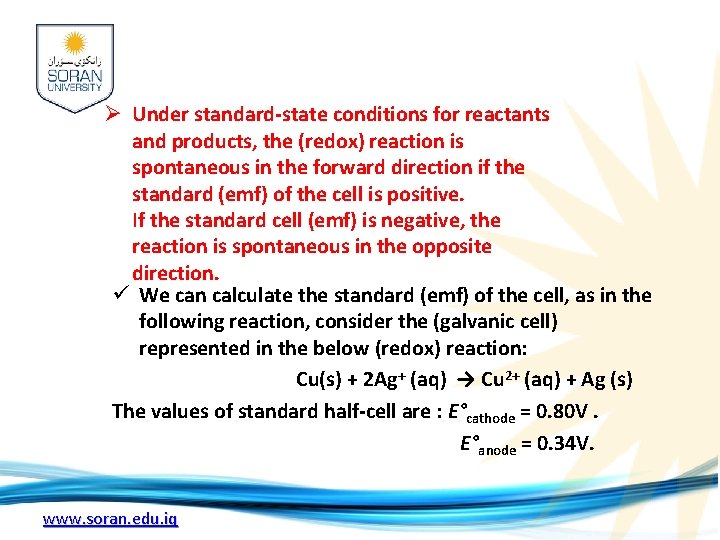
Ø Under standard-state conditions for reactants and products, the (redox) reaction is spontaneous in the forward direction if the standard (emf) of the cell is positive. If the standard cell (emf) is negative, the reaction is spontaneous in the opposite direction. ü We can calculate the standard (emf) of the cell, as in the following reaction, consider the (galvanic cell) represented in the below (redox) reaction: Cu(s) + 2 Ag+ (aq) → Cu 2+ (aq) + Ag (s) The values of standard half-cell are : E°cathode = 0. 80 V. E°anode = 0. 34 V. www. soran. edu. iq

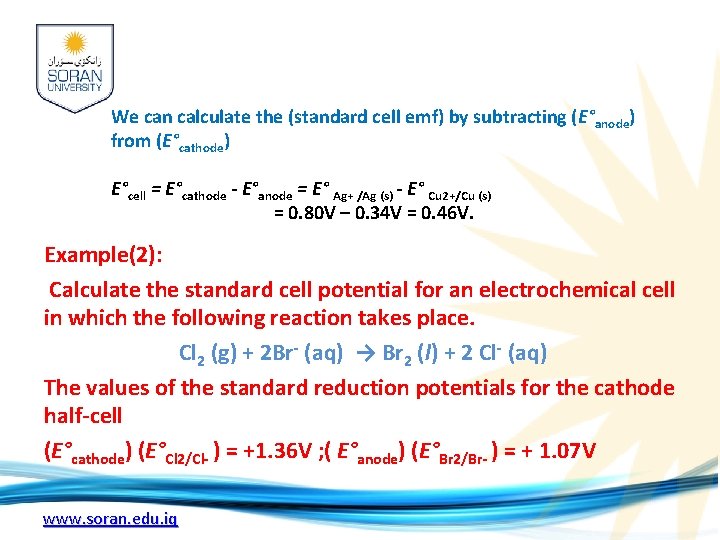
Solution: Let's break this reaction down into its two half-reactions. Cu(s) Cu 2+ (aq) + 2 e-. 2 Ag+ (aq) + 2 e- 2 Ag (s) We can calculate the (standard cell emf) by subtracting (E°anode) from (E°cathode) E°cell = E°cathode - E°anode = E° Ag+ /Ag (s) - E° Cu 2+/Cu (s) = 0. 80 V – 0. 34 V = 0. 46 V. www. soran. edu. iq

We can calculate the (standard cell emf) by subtracting (E°anode) from (E°cathode) E°cell = E°cathode - E°anode = E° Ag+ /Ag (s) - E° Cu 2+/Cu (s) = 0. 80 V – 0. 34 V = 0. 46 V. Example(2): Calculate the standard cell potential for an electrochemical cell in which the following reaction takes place. Cl 2 (g) + 2 Br- (aq) → Br 2 (l) + 2 Cl- (aq) The values of the standard reduction potentials for the cathode half-cell (E°cathode) (E°Cl 2/Cl- ) = +1. 36 V ; ( E°anode) (E°Br 2/Br- ) = + 1. 07 V www. soran. edu. iq

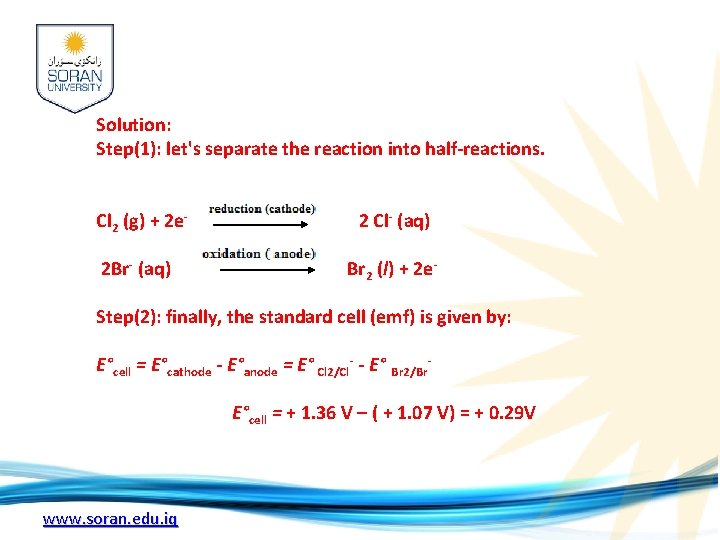
Solution: Step(1): let's separate the reaction into half-reactions. Cl 2 (g) + 2 e- 2 Cl- (aq) 2 Br- (aq) Br 2 (l) + 2 e Step(2): finally, the standard cell (emf) is given by: E°cell = E°cathode - E°anode = E° Cl 2/Cl- - E° Br 2/Br E°cell = + 1. 36 V – ( + 1. 07 V) = + 0. 29 V www. soran. edu. iq

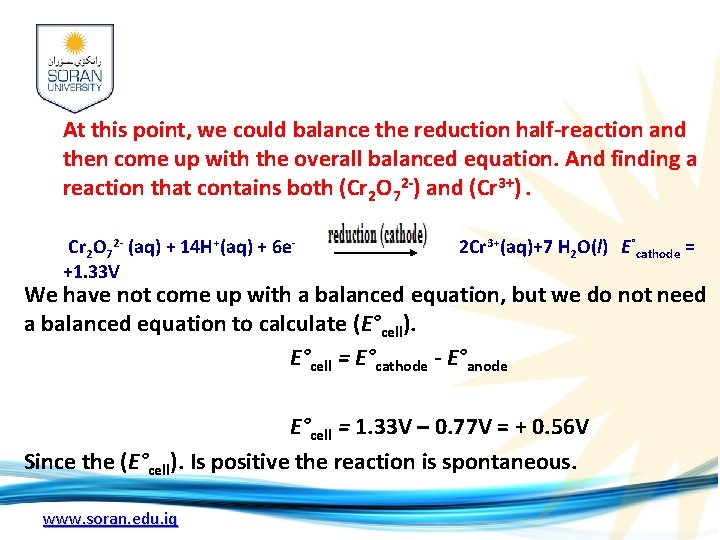
At this point, we could balance the reduction half-reaction and then come up with the overall balanced equation. And finding a reaction that contains both (Cr 2 O 72 -) and (Cr 3+). Cr 2 O 72 - (aq) + 14 H+(aq) + 6 e- 2 Cr 3+(aq)+7 H 2 O(l) E°cathode = +1. 33 V We have not come up with a balanced equation, but we do not need a balanced equation to calculate (E°cell). E°cell = E°cathode - E°anode E°cell = 1. 33 V – 0. 77 V = + 0. 56 V Since the (E°cell). Is positive the reaction is spontaneous. www. soran. edu. iq













