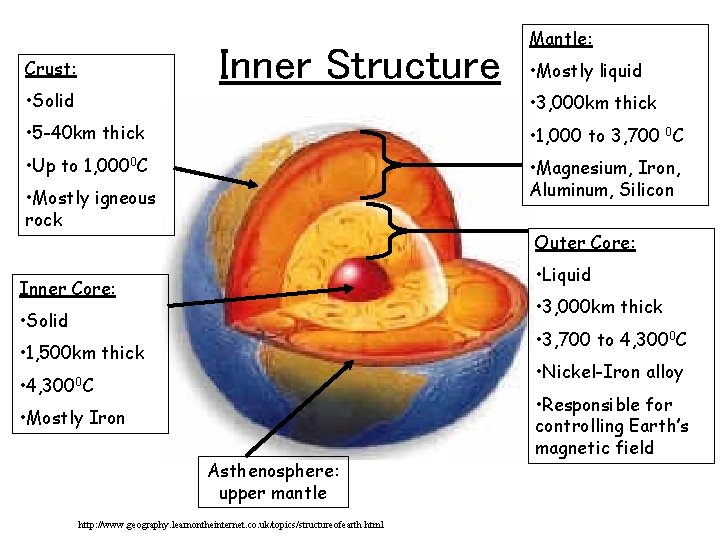
Inner Structure Crust Mantle Mostly liquid Solid 3




















![References • Internet Geography [not date] Retrieved form the site: http: //www. geography. learnontheinternet. References • Internet Geography [not date] Retrieved form the site: http: //www. geography. learnontheinternet.](https://slidetodoc.com/presentation_image_h2/77c5c001636487326a5b97c0b2ca60ff/image-29.jpg)
- Slides: 29


Inner Structure Crust: Mantle: • Mostly liquid • Solid • 3, 000 km thick • 5 -40 km thick • 1, 000 to 3, 700 0 C • Up to 1, 0000 C • Magnesium, Iron, Aluminum, Silicon • Mostly igneous rock Outer Core: • Liquid Inner Core: • 3, 000 km thick • Solid • 3, 700 to 4, 3000 C • 1, 500 km thick • Nickel-Iron alloy • 4, 3000 C • Mostly Iron Asthenosphere: upper mantle http: //www. geography. learnontheinternet. co. uk/topics/structureofearth. html • Responsible for controlling Earth’s magnetic field

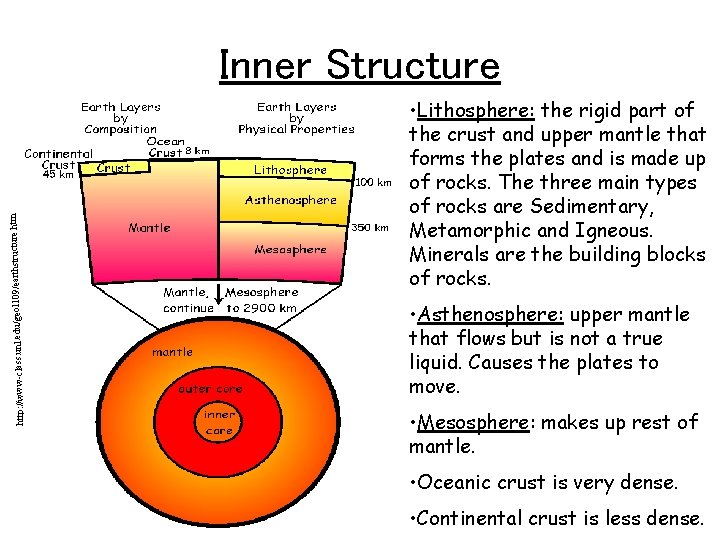
structure. htm http: //www-class. unl. edu/geol 109/earth Inner Structure • Lithosphere: the rigid part of the crust and upper mantle that forms the plates and is made up of rocks. The three main types of rocks are Sedimentary, Metamorphic and Igneous. Minerals are the building blocks of rocks. • Asthenosphere: upper mantle that flows but is not a true liquid. Causes the plates to move. • Mesosphere: makes up rest of mantle. • Oceanic crust is very dense. • Continental crust is less dense.

Sedimentary Rocks • Sedimentary rocks are formed when layers of sediment are deposited at the bottom of seas and lakes. • Over millions of years, the layers underneath become squashed by the layers on top. • The water is squeezed out of the bottom layers so that the layers become cemented together. • Examples of sedimentary rock include: • Sandstone – hardened sand • Mudstone – hardened mud • Shale – hardened mud • Conglomerate – pebbles and other debris cemented together • Limestone – made from lime (calcium carbonate) deposits from shells. Stalagmites and stalactites found in caves are limestone.

Sedimentary Rock & • Fossil fuels such as coal, oil and natural gas are formed in sedimentary rock. • Hundreds of millions of years ago the dead organisms bodies became trapped in the sedimentary layers. • When the layers became deep enough, the intense heat and pressure caused chemical reactions in the dead organic matter and turned it into hydrocarbons. • Humans have learnt to extract these fossil fuels from deep within the Earth and burn them in order to provide energy. http: //www. energyquest. ca. gov/story/chapter 08. html http: //members. macconnect. com/users/d/dansy monds/My%20 Army%20 Life. html http: //www. nicorinc. com/en_us/commercial/product s_and_services/natural_gas_cooking. htm

Metamorphic Rocks • Metamorphic rock is formed as sedimentary rock is pushed deeper into the Earth’s surface. • Here it is heated due to the pressure from rocks layered above it and the surrounding temperatures in the mantle. • The temperature changes the chemical composition of the rock. • Sedimentary shale becomes metamorphic slate. • Sedimentary limestone becomes marble which is a very hard material and has many uses in our everyday lives.

Igneous Rocks • Igneous rocks are formed when molten rock (magma if it is below the surface and lava if it is above the surface) solidifies. • Igneous rock has crystals in it. The crystals are small if the rock has cooled quickly and large if the rock has cooled slowly. • Examples include: • Granite • Pumice • Basalt

Weathering & Erosion • Weathering is the process of breaking down rocks. • This can either be physical weathering or chemical weathering. • Physical Weathering just breaks down the rocks into smaller pieces without altering the chemical structure. • Chemical weathering breaks down the rocks by chemical reactions forming new compounds • Erosion is the movement of weathered particles from one place to another. Kimberley Region north Western Australia

Agents of Weathering and Erosion Surfers Paradise, Queensland Agents of Weathering • Wind • Waves • Running Water • Extremes of heat and cold • Acid • Tree roots Sometimes the agents cause weathering and erosion at the same time Agents of Erosion • Wind • Waves • Running Water • Glaciers

Wind Bondi Cliffs Kimberley Region north Western Australia The wind picks up sand particles acting like sand paper, weathering and eroding the rocks

Waves The Gap, Sydney The Great Australian Bight South Australia The energy of the waves causes parts of the cliff to be broken off and then the waves wash the weathered material away. The cliffs are both weathered and eroded by the waves

Waves The 12 Apostles Southern Coastline of Victoria All of these rocks were once part of the mainland the energy of the waves has weathered and eroded the rocks producing the 12 Apostles, caves and beaches.

Running Water Mungo National Park south west of NSW, near the Victorian border The rain weathers and erodes the rocks to form the patterns shown.

Running Water Small waterfall in the Kimberly Region in north Western Australia Running water causes both the wearing away of rocks, making them very rounded and smooth and the erosion of the worn away pieces

Running Water The rain dissolves chemicals in the rock causing chemical weathering and erosion of the material and producing vertical cracks.

Chemical Weathering Jenolan Caves NSW Running water dissolves calcium compounds in the rocks which are then washed away leaving large caves. As the water evaporates from droplets stalagmites and stalactites form.

Acid & Tree Roots Physical Weathering: The roots growing in the cracks cause the cracks to become larger until eventually pieces of rock break off. Chemical Weathering: The acid produced by the roots chemically breaks down the surrounding rock.

Different types of rocks weather and erode at different rates “China Wall” in the Kimberley Region. The “wall” left standing is a quartz outcrop Ayres Rock or Uluru, central Australia Weathering and erosion caused this huge rock to become exposed and stand above its surroundings.

State the agents of weathering & erosion for A & B A. Rocks between Bondi & Bronte B. Rocks along a river in the Kimberley Region in north Western Australia

State the agents of weathering & erosion for C & D C. Kimberly Region north West Australia D. Kimberly Region north West Australia

State the agents of weathering & erosion for E & F E The Bungles in the Kimberly Region F

Stopping Erosion Plants in the sand or soil stop the sand or soil from being washed or blown away and therefore stop the erosion

Deposition Small particles eroded upstream are deposited on the slow bend of the river and as small islands in the center shallows. http: //www. kented. org. uk/ngfl/rivers/River%20 Articles/braiding. htm

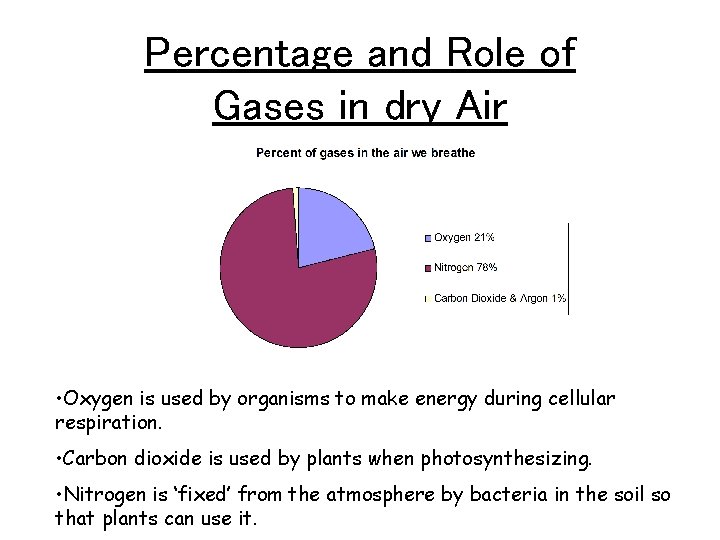
Percentage and Role of Gases in dry Air • Oxygen is used by organisms to make energy during cellular respiration. • Carbon dioxide is used by plants when photosynthesizing. • Nitrogen is ‘fixed’ from the atmosphere by bacteria in the soil so that plants can use it.

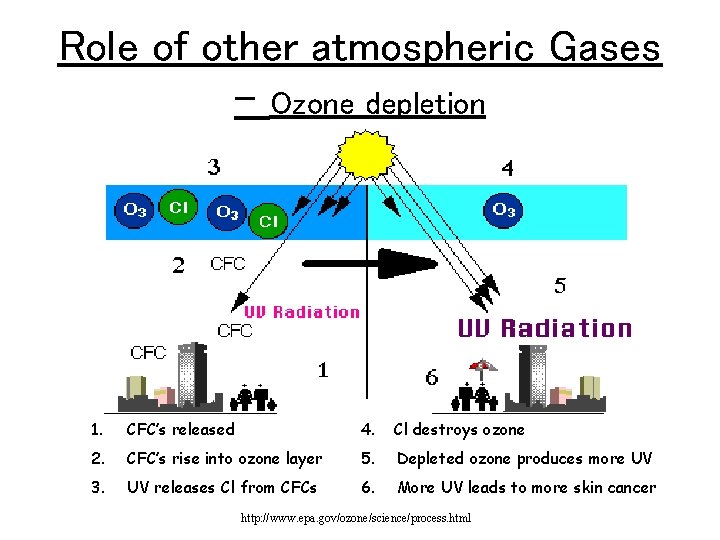
Role of other atmospheric Gases – Ozone depletion 1. CFC’s released 4. Cl destroys ozone 2. CFC’s rise into ozone layer 5. Depleted ozone produces more UV 3. UV releases Cl from CFCs 6. More UV leads to more skin cancer http: //www. epa. gov/ozone/science/process. html

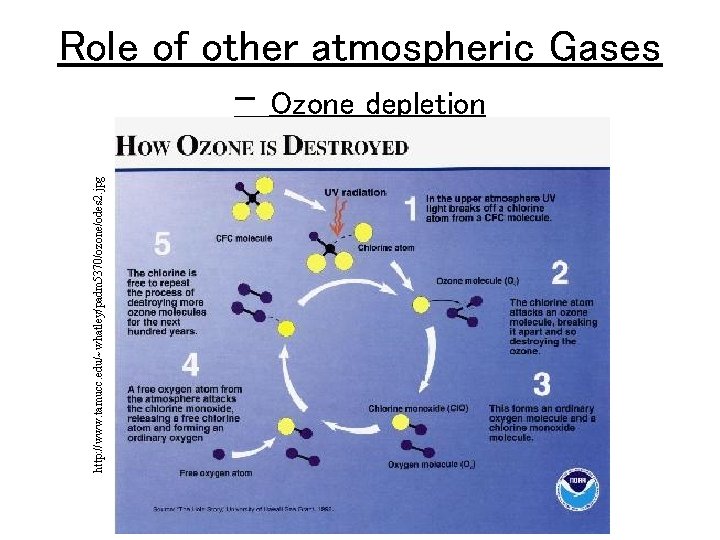
http: //www. tamucc. edu/~whatley/padm 5370/ozone/odes 2. jpg Role of other atmospheric Gases – Ozone depletion

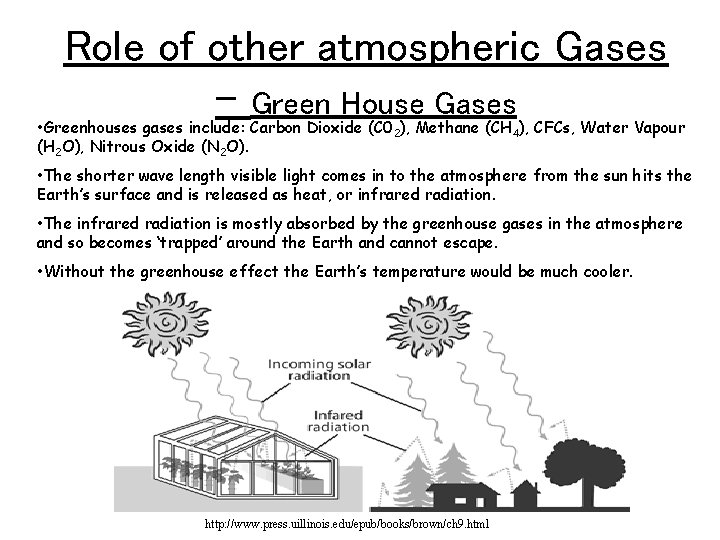
Role of other atmospheric Gases – Green House Gases • Greenhouses gases include: Carbon Dioxide (C 02), Methane (CH 4), CFCs, Water Vapour (H 2 O), Nitrous Oxide (N 2 O). • The shorter wave length visible light comes in to the atmosphere from the sun hits the Earth’s surface and is released as heat, or infrared radiation. • The infrared radiation is mostly absorbed by the greenhouse gases in the atmosphere and so becomes ‘trapped’ around the Earth and cannot escape. • Without the greenhouse effect the Earth’s temperature would be much cooler. http: //www. press. uillinois. edu/epub/books/brown/ch 9. html

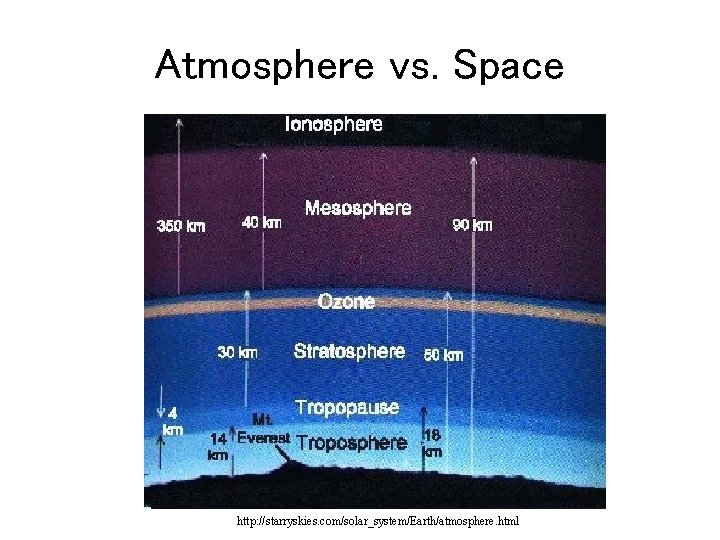
Atmosphere vs. Space http: //starryskies. com/solar_system/Earth/atmosphere. html
![References Internet Geography not date Retrieved form the site http www geography learnontheinternet References • Internet Geography [not date] Retrieved form the site: http: //www. geography. learnontheinternet.](https://slidetodoc.com/presentation_image_h2/77c5c001636487326a5b97c0b2ca60ff/image-29.jpg)
References • Internet Geography [not date] Retrieved form the site: http: //www. geography. learnontheinternet. co. uk/topics/structureofearth. html September 2004. • Miles, K. & Peters, C. (1997) Earth’s Atmosphere. Retrieved from the site: http: //starryskies. com/solar_system/Earth/atmosphere. html September 2004 • Ritter, M. (2003) The Physical Environment. Retrieved from the site: http: //www. uwsp. edu/geo/faculty/ritter/glossary/l_n/lithosphere. html September 2004. • http: //www-class. unl. edu/geol 109/earthstructure. htm ddd • Moorland School Clitheroe [no date] Earth Science - The Rock Cycle. Retrieved from the site: http: //www. moorlandschool. co. uk/earth/rockcycle. htm September 2004. • United States Environment protection Agency (2004) The Process of Ozone Depletion. Retrieved from the site: http: //www. epa. gov/ozone/science/process. html October 2004. • University of Illinois (2003) Global Warming. Retrieved from the site: http: //www. press. uillinois. edu/epub/books/brown/ch 9. html October 2004.