Phase Changes Solids Liquids and Gases 2 3












- Slides: 16

Phase Changes: (Solids, Liquids and Gases) 2. 3. 1 -2. 3. 2

Daily Demo Boo Bubbles

Dry Ice Balloons 1. Dry ice is solid Carbon Dioxide. Observe the changes to a balloon with dry ice placed inside. What happens? 2. What are the CO 2 molecules doing during this process? Sketch your hypothesis. 3. Do gaseous CO 2 molecules have more or less energy than solid CO 2 molecules. Explain your answer.

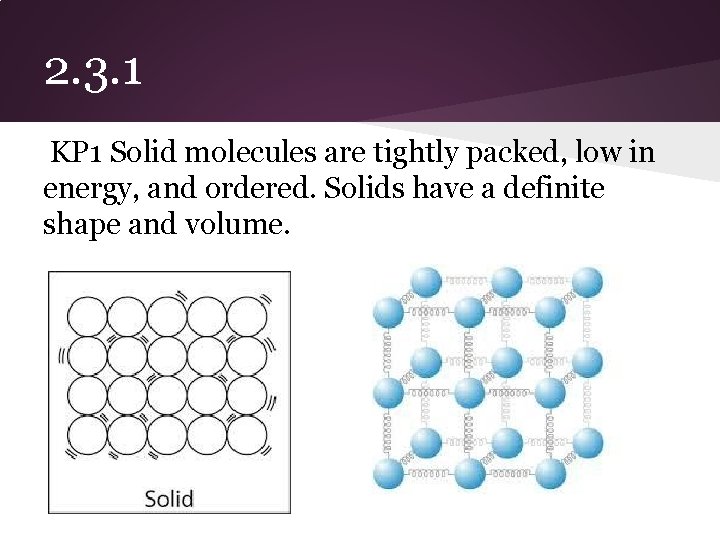
2. 3. 1 KP 1 Solid molecules are tightly packed, low in energy, and ordered. Solids have a definite shape and volume.

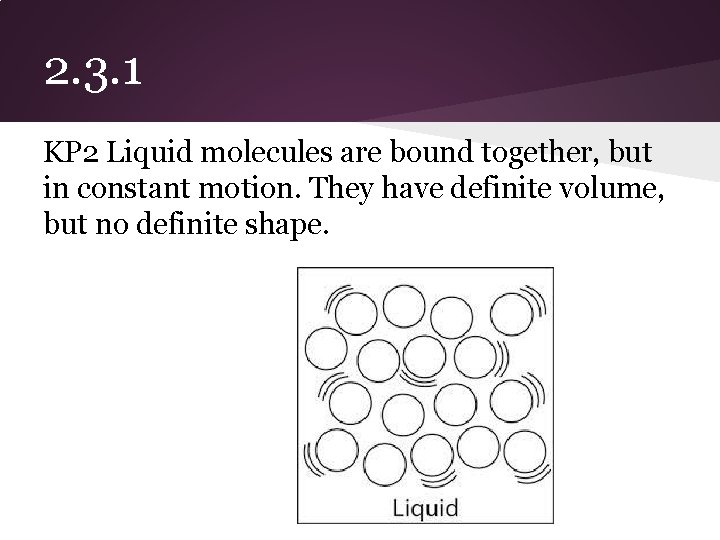
2. 3. 1 KP 2 Liquid molecules are bound together, but in constant motion. They have definite volume, but no definite shape.

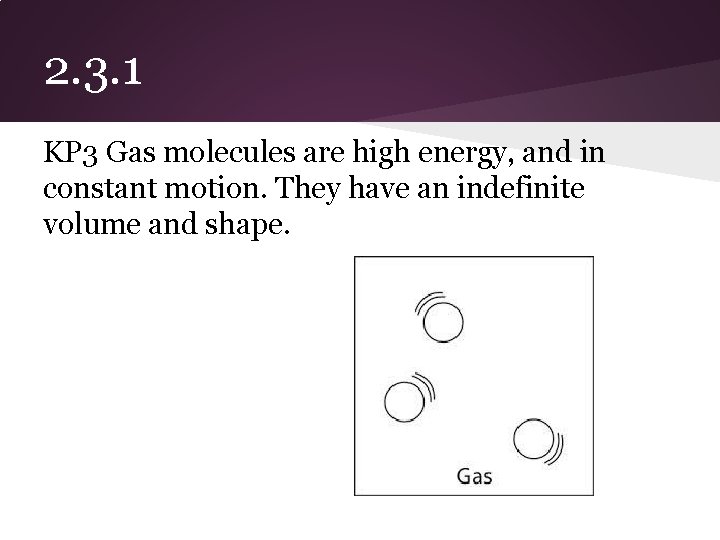
2. 3. 1 KP 3 Gas molecules are high energy, and in constant motion. They have an indefinite volume and shape.

We Do- Gizmo http: //www. explorelearning. com/index. cfm? m ethod=c. Resource. dsp. Detail&Resource. ID=661

You Together - Water Ice 1. Observe the water ice melting in your cup. What different types, or “states”, of matter do you observe? 2. How would you describe the motions of water molecules during these different “states”? 3. Sketch your ideas below.

You Alone 1. Read Pages 340 -348 in a textbook silently. 2. Silently answer questions 11 -12, 15 -17 on pages 346 and 348 on a blank sheet of paper.

Closing

Exit Ticket 1. A solid is a state of matter that has a(n) a. indefinite volume and an indefinite shape. b. definite volume and a definite shape. c. definite volume and an indefinite shape. d. indefinite volume and a definite shape.

2. The change from solid to liquid is called: A. Sublimation B. Deposition C. Freezing D. Melting

3. In which state of matter are particles packed tightly together in a fixed position? A. Compound B. Solid C. Liquid D. Gas

4. In which state of matter do the particles spread out and fill all the space that is available to them? A. Solid B. Liquid C. Gas D. Compound

5. During the process of sublimation A. A solid turns directly into a gas B. A gas turns directly into a solid. C. A solid turns into a liquid D. A liquid turns into a solid.

Review Standard 1 Hydrosphere - Water Cycle -SC. 6. E. 7. 2: Investigate and apply how the cycling of water between the atmosphere and the hydrosphere has an effect on weather patterns and climate.