Immunization of Health Care Workers more than just





























- Slides: 43

Immunization of Health Care Workers: more than just influenza Mary Vearncombe, MD, FRCPC Medical Director, Infection Prevention & Control Sunnybrook Health Sciences Centre, Toronto

Disclosure No conflict of interest to disclose.

Objectives: 1. 2. 3. To identify vaccine preventable diseases relevant to hospital occupational health To determine HCW susceptibility/immunity To determine appropriate immunization and serology

HCW Immunization: Background HCWs are at risk of exposure to and possible transmission of communicable diseases - some are vaccine preventable establishing and maintaining immunity is an essential component of both Occupational Health and Infection Prevention and Control programs

HCW Immunization: Background applies to all health care settings ◦ offices, clinics, acute care, LTC, laboratories, first responders, etc. applies to all health care personnel ◦ employees, physicians, students, contract workers, volunteers ◦ student immunization should occur before clinical placement

HCW Immunization: Background immunization protects HCWs, their families, colleagues and patients cost containment through prevention of infection furloughing susceptible HCWs after exposure costs of prophylaxis costs of treatment absenteeism during acute illness disability following illness outbreak investigation and control

HCW Immunization active immunization strongly recommended - specific risk for HCWs ◦ i. e. , hepatitis B, annual influenza, measles, mumps, rubella, varicella, acellular pertussis immunization recommended for all adults ◦ i. e. , tetanus, diphtheria

Occupational Health Assessment Before placement health inventory: ◦ immunization status ◦ to guide: further immunizations, post -exposure management ◦ opportunity for adult immunization in immigrant HCWs education: ◦ importance of maintaining personal health – healthy workplace ◦ need for annual influenza vaccine

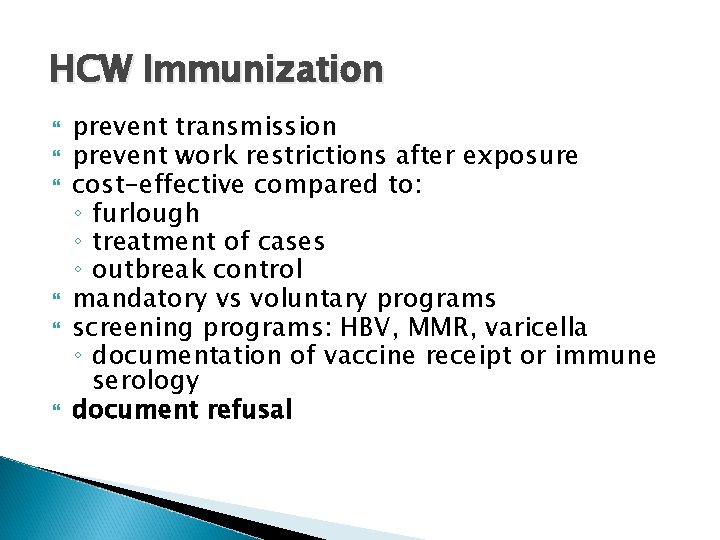
HCW Immunization prevent transmission prevent work restrictions after exposure cost-effective compared to: ◦ furlough ◦ treatment of cases ◦ outbreak control mandatory vs voluntary programs screening programs: HBV, MMR, varicella ◦ documentation of vaccine receipt or immune serology document refusal

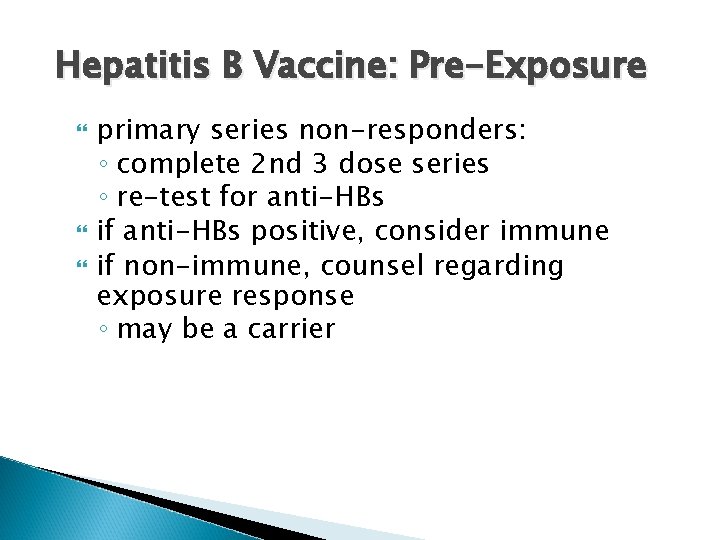
Hepatitis B Vaccine: Pre-Exposure Pre-placement: HB vaccine for all HCWs at risk of exposure to hepatitis B, i. e. , who may have contact with blood, body fluids or sharps risk often highest during training period ◦ vaccination should be completed during training, before clinical exposure test for anti-HBs 1 month after vaccine series complete

Hepatitis B Vaccine: Pre-Exposure primary series non-responders: ◦ complete 2 nd 3 dose series ◦ re-test for anti-HBs if anti-HBs positive, consider immune if non-immune, counsel regarding exposure response ◦ may be a carrier

Hepatitis B Vaccine: Ongoing Surveillance periodic antibody testing not recommended booster doses not recommended HBV unimmunized or non-responders to vaccine at risk for exposure should be offered annual screening for HBs. Ag ◦ assessment and treatment ◦ protection of partner and household contacts

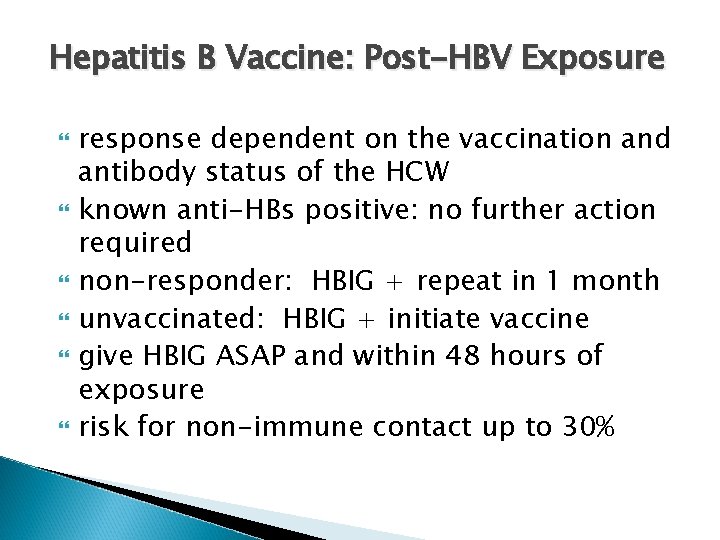
Hepatitis B Vaccine: Post-HBV Exposure response dependent on the vaccination and antibody status of the HCW known anti-HBs positive: no further action required non-responder: HBIG + repeat in 1 month unvaccinated: HBIG + initiate vaccine give HBIG ASAP and within 48 hours of exposure risk for non-immune contact up to 30%

Influenza Vaccine NACI Recommended Recipients “People capable of transmitting influenza to those at high risk for influenzarelated complications” ◦ All health care workers: acute care, long term care, home care and outpatient settings

Influenza Vaccine Why should I be immunized? You will protect yourself from acquiring “the flu”, or if you do get “the flu” it will be less severe. Influenza vaccine is effective in otherwise healthy adults. ◦ NEJM 333: 14 889 -893, 1995 ◦ JAMA 281: 10 908 -913, 1999 ◦ JAMA 284: 13 1655 -1663, 2000

Influenza Vaccine Why should I be immunized? You will protect your patients from influenza. Vaccination of HCWs reduces illness and mortality of frail elderly patients more effectively than vaccination of patients. ◦ ◦ JID 175: 1 -6, 1997 Lancet 355: 8/1/ 2000, 93 -97 BMJ 333(7581): 1241, 2006 J Am Ger Soc 57(9): 1580 -6, 2009

Influenza Vaccine Pre-placement: counsel with regard to implications of transmission of respiratory viruses to patients ◦ healthy workplace counsel with regard to expectation of annual influenza immunization pregnancy is an indication, not a contraindication, for influenza vaccine

Influenza Vaccine Ongoing Surveillance: recommend influenza vaccine annually to all HCWs before the beginning of the influenza season ◦ The advice of a health care professional is an important factor in acceptance of vaccine utilize strategies to maximize vaccine coverage ◦ e. g. , mobile carts, shift coverage, education, incentives, peer immunization, declination forms ◦ mandatory immunization?

Influenza Vaccine Influenza outbreaks: immunized personnel may continue to work unimmunized personnel working in the affected unit must take antiviral chemoprophylaxis for 2 weeks if they also receive vaccine or until end of outbreak unimmunized personnel who refuse chemoprophylaxis should not provide patient care

Annual Influenza Immunization National Advisory Committee on Immunization Public Health Agency of Canada “The provision of influenza vaccination for HCWs involved in direct patient care is an essential component of the standard of care for influenza prevention. ” “HCWs involved in direct patient care should consider it their responsibility to provide the highest standard of care, which includes annual influenza vaccination. ” “In the absence of contraindications, refusal of HCWs who are involved in direct patient care to be immunized against influenza implies failure in their duty of care to their patients. ”

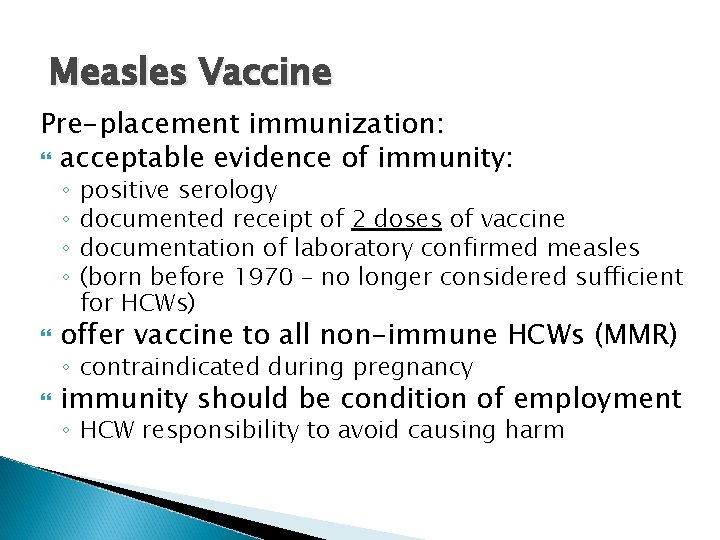
Measles Vaccine Pre-placement immunization: acceptable evidence of immunity: ◦ ◦ positive serology documented receipt of 2 doses of vaccine documentation of laboratory confirmed measles (born before 1970 – no longer considered sufficient for HCWs) offer vaccine to all non-immune HCWs (MMR) immunity should be condition of employment ◦ contraindicated during pregnancy ◦ HCW responsibility to avoid causing harm

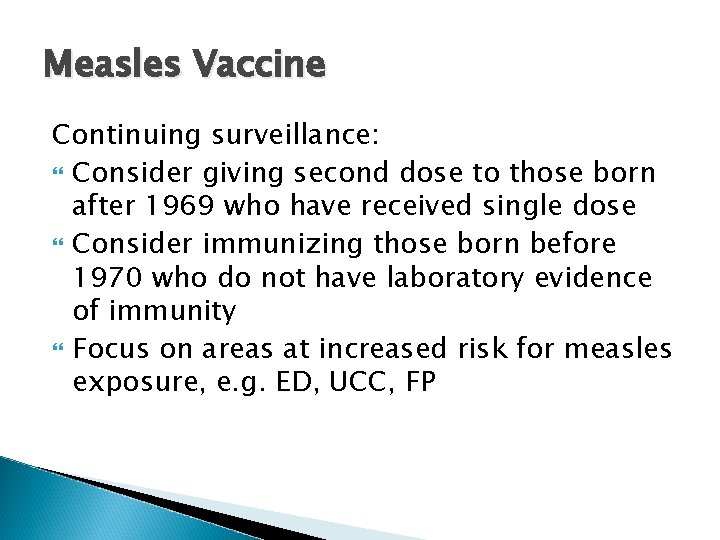
Measles Vaccine Continuing surveillance: Consider giving second dose to those born after 1969 who have received single dose Consider immunizing those born before 1970 who do not have laboratory evidence of immunity Focus on areas at increased risk for measles exposure, e. g. ED, UCC, FP

Measles Vaccine Post-Exposure: immunization of susceptible person within 72 hours of exposure usually prevents measles ◦ still require furlough

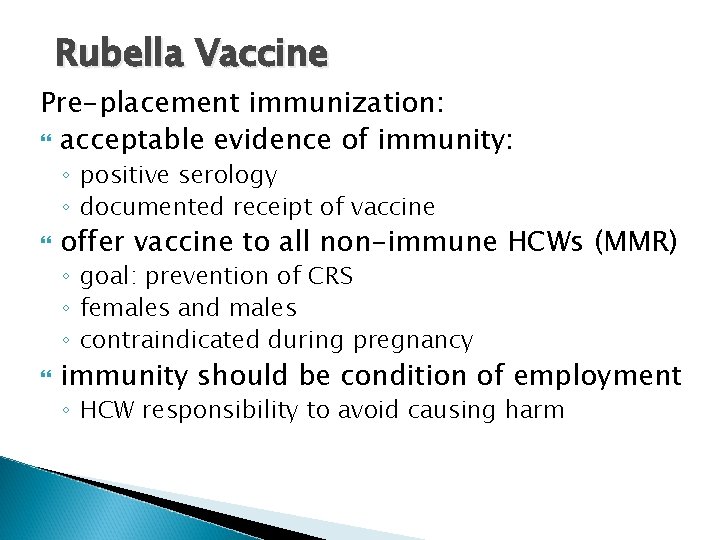
Rubella Vaccine Pre-placement immunization: acceptable evidence of immunity: ◦ positive serology ◦ documented receipt of vaccine offer vaccine to all non-immune HCWs (MMR) ◦ goal: prevention of CRS ◦ females and males ◦ contraindicated during pregnancy immunity should be condition of employment ◦ HCW responsibility to avoid causing harm

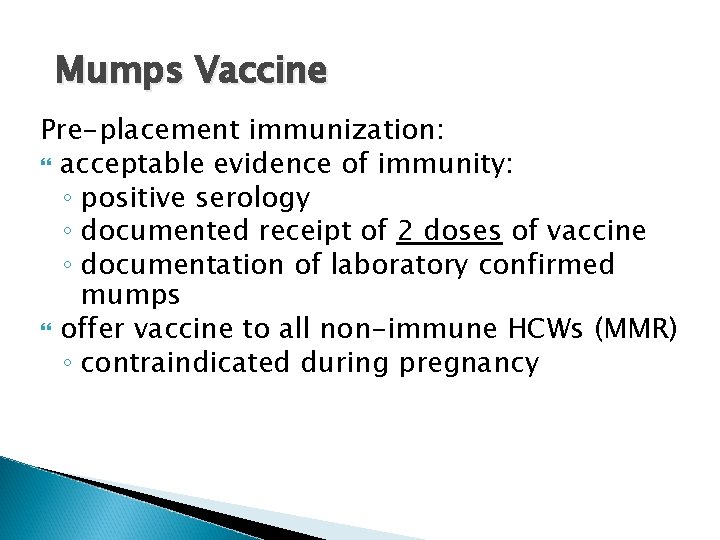
Mumps Vaccine Pre-placement immunization: acceptable evidence of immunity: ◦ positive serology ◦ documented receipt of 2 doses of vaccine ◦ documentation of laboratory confirmed mumps offer vaccine to all non-immune HCWs (MMR) ◦ contraindicated during pregnancy

Mumps Vaccine Continuing surveillance: Consider giving second dose to those born after 1969 who have received single dose Consider immunizing those born before 1970 who do not have laboratory evidence of immunity Focus on areas at increased risk for mumps exposure, e. g. ED, UCC, FP

Mumps Vaccine Post-Exposure: mumps immunization after exposure may not prevent disease, but will confer protection against future exposures

Varicella Vaccine Pre-placement: ascertain history of varicella/zoster ◦ definite history: assume immune ◦ negative or uncertain: antibody screen offer vaccine (2 doses) to HCWs who are non -immune ◦ contraindicated during pregnancy ◦ post-vaccine serology not recommended: high efficacy of vaccine commercially available tests not sufficiently sensitive for post-vaccine immunity

Varicella Vaccine Adverse Events: Post-vaccine rash: ◦ at injection site: cover and may continue to work ◦ non-injection site: small number of papules/ vesicles and low grade fever - should not work with high-risk patients, e. g. newborns, obstetrics, transplants, oncology ◦ Note: varicelliform rashes within 2 weeks of vaccine are usually due to wild-type virus

Varicella Vaccine Post-Exposure management of vaccine recipients: vaccine offers 70 - 90% protection against varicella; 95% protection against severe varicella observe daily at start of shift for signs/ symptoms of varicella day 10 to 21

Varicella Vaccine Post-exposure vaccine use: vaccine may prevent or reduce severity of varicella if given within 72 hours of exposure furlough still required day 10 to 21 immunity for subsequent exposures outbreak control

Pertussis Vaccine pertussis is a frequent cause of cough illness in adolescents and adults; major reservoir of disease and source of transmission nosocomial transmission to both patients and HCWs occurs prevention of secondary cases difficult as symptoms are non-specific and diagnosis difficult during catarrhal stage a single dose of Tdap should be offered to all HCWs who have not received an adolescent/adult dose

Meningococcal Disease Occupational Risk in Clinical HCWs: There is no risk to HCWs from casual contact with patients with meningococcal disease Transmission to HCWs from patients with invasive meningococcal disease may occur after intensive, direct contact where the patient’s respiratory secretions contaminate the HCW’s oral/nasal mucous membranes, e. g. intubation, airway management, suctioning, close examination of oropharynx, when facial protection not worn

Meningococcal Disease Occupational Risk in Microbiology Laboratory Technologists several reports of invasive infection in technologists no identified breaches in laboratory technique many cases fatal rate of disease in microbiology laboratory technologists dealing with N. meningitidis cultures elevated (US, UK)

Meningococcal Vaccine: NACI routine vaccination of healthcare workers not currently recommended ◦ antibiotic chemoprophylaxis sufficient if exposure occurs research, industrial and clinical laboratory personnel who are routinely exposed to N. meningitidis cultures: ◦ quadrivalent A, C, Y, W-135 conjugate vaccine ◦ vaccine does not replace laboratory safety standards: serogropup B not in vaccine

Hepatitis A Vaccine Pre-placement: routine use of vaccine not recommended ◦ HCWs not at increased risk ◦ routine infection control practices prevent transmission counsel re prevention of transmission, i. e. , hand hygiene; no eating, drinking, in patient care areas

Hepatitis A Vaccine Post-Exposure/Outbreak Control: give vaccine for post-exposure prophylaxis as soon as possible and within 7 days of exposure ◦ not required for routine care of patients with hepatitis A

BCG Vaccine BCG vaccine does not provide permanent or absolute protection against TB loss of TST as marker of infection BCG vaccination of HCWs, including MLTs, may be considered when all of the following exist: ◦ there is a considerable risk of exposure/ transmission of tubercle bacilli ◦ a high percentage of strains are drug-resistant ◦ infection control measures have been ineffective or are not feasible

Tetanus/Diphtheria Vaccine Pre-placement: immunization history ◦ immigrant HCWs may not have received primary immunization series maintain immunity with booster Td (tetanus/diphtheria toxoid) every 10 years ◦ One dose with acellular pertussis (Tdap); do not need to wait until next booster due

Routine Vaccines Strongly Recommended for HCWs Hepatitis B vaccine Annual Influenza vaccine Measles/Mumps/Rubella vaccine (MMR) Varicella vaccine Acellular Pertussis (Tdap) Meningococcal vaccine for microbiology MLTs Tetanus/Diphtheria vaccine (Td)

HCWs and Vaccine Preventable Diseases HCWs are at risk for acquiring infections from patients and, if infected, transmitting infections to patients and initiating or propagating outbreaks The most effective way to prevent vaccine preventable diseases is by ensuring immunity ◦ Start with pre-clinical health care students Susceptible HCWs should be immunized with the appropriate vaccine(s) unless there is a medical contraindication ◦ Personal belief systems against vaccines are not acceptable when patient safety is at risk The advice of a health care professional – your advice - is an important factor in vaccine acceptance

Essential References 1. 2. ◦ Canadian Immunization Guide, 7 th edition, 2006, National Advisory Committee on Immunization Recommendations, Public Health Agency of Canada OHA/OMA/MOHLTC Communicable Disease Surveillance Protocols www. oha. com → Disease Protocols

 More more more i want more more more more we praise you
More more more i want more more more more we praise you More more more i want more more more more we praise you
More more more i want more more more more we praise you Software is more than just a
Software is more than just a Bingo bins mortdale
Bingo bins mortdale Math is more than just numbers
Math is more than just numbers It's more than just rain or snow
It's more than just rain or snow It's more than just oil slogan
It's more than just oil slogan Generous kelimesinin comparative hali
Generous kelimesinin comparative hali 5730x5
5730x5 Better than god
Better than god Nothing is more precious than health
Nothing is more precious than health Primary, secondary, tertiary care
Primary, secondary, tertiary care Why is it difficult to get poison in mantua?
Why is it difficult to get poison in mantua? Unit 2 equality diversity and rights
Unit 2 equality diversity and rights Paano nakakahawa ang leptospirosis
Paano nakakahawa ang leptospirosis Basic training for barangay health workers
Basic training for barangay health workers Basic training for barangay health workers
Basic training for barangay health workers Basic training for barangay health workers
Basic training for barangay health workers C.d.c. covid isolation health workers
C.d.c. covid isolation health workers Covid isolation health workers
Covid isolation health workers C.d.c. covid isolation health workers
C.d.c. covid isolation health workers Covid isolation health workers
Covid isolation health workers Community health workers
Community health workers Personal traits for health informatics services workers
Personal traits for health informatics services workers Occupational health clinic for ontario workers
Occupational health clinic for ontario workers Health and social component 3
Health and social component 3 A grosso has a group of soloists rather
A grosso has a group of soloists rather Just in case bag palliative care
Just in case bag palliative care Mersey care just culture
Mersey care just culture Percents less than 1
Percents less than 1 Greater than less than fractions
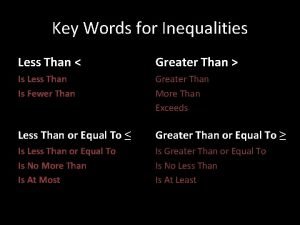
Greater than less than fractions Key words for inequality signs
Key words for inequality signs Odd one out fractions
Odd one out fractions Jesus lord of heaven
Jesus lord of heaven Numberblocks 10
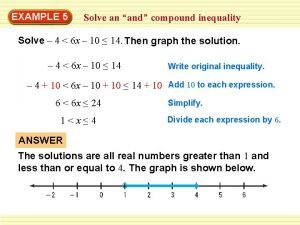
Numberblocks 10 Compound inequality definition
Compound inequality definition Sentences with more than
Sentences with more than Curriculum evaluation definition
Curriculum evaluation definition Pda is less powerful than turing machines
Pda is less powerful than turing machines Can an atom have more neutrons than protons
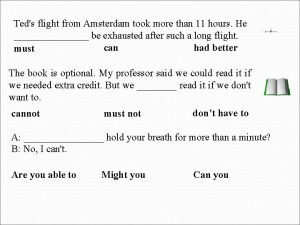
Can an atom have more neutrons than protons Ted's flight from amsterdam
Ted's flight from amsterdam How to cite more than one author
How to cite more than one author How to cite more than 3 authors
How to cite more than 3 authors Inequality open circle or closed
Inequality open circle or closed