History of Pharmacy Origin history development of pharmacy
































- Slides: 32

History of Pharmacy ØOrigin, history & development of pharmacy in India. ØPharmacy in relation to allied health profession. ØPharmacopoeias – I. P. , B. P. , U. S. P, and EP

Origin, history & development of pharmacy • Ayurveda • Ayu (Life) • Veda (Knowledge) • Charak-samhita is a continuation & renewal of ancient knowledge. • Sushrutha-sanhita- encyclopedia of surgery

Origin, history & development of pharmacy contd. • Archaeological survey discovered the material related to the historical part of drugs or medicines, Papyrus. Ebers of 16 th Century BC. • It contains important information on vegetable drugs & the formulations made from them.

Origin, history & development of pharmacy contd. • Then came the era of individuals by virtue of their involvement in the profession, contributed for development in science, which in turn has affected pharmacy profession. • Hippocrates • Aristotle • Dioscorides • Galen etc

Hippocrates- Father of Medicine (466 -377 BC) • Systematized the knowledge available and linked it with ethics • His knowledge were then accepted worldwide & still utilized by health professionals. • He developed an Oath of Medical Ethics for physicians to follow. • Hippocrates is known as the "Father of Medicine".

Aristotle (384/3 B. C. -322/1 B. C. ) • He not only contributed enormously to medicine per se, but also to the natural sciences, for he was the first to classify animals • It seems that he sustained the notion that certain "inferior" animals such as insects (whose name derived from the evident segmentation of the body into its components) came into the world spontaneously from decomposing material regenerating and so their growth could not be limited or restrained. • Aristotle elaborated a physiological system centred on the heart, in which according to him, there burned a life-giving vital flame maintained by a spirit named pneuma or spirito vitale (vital spirit) that produced heat. He felt that the lungs and the brain had a primary function of cooling. The heart was the most important organ because when the heart stopped, the body died. • Furthermore, in his studies of embryology. • In his theory, heat was the most important thing and gave life. He sustained that man, having a great deal of heat, managed to use all his body's resources and to produce sperm. On the other hand, women did not have enough heat so a part of the body's blood was eliminated during menstruation. With its heat, sperm acted on menstruation, producing the embryo.

Dioscorides - A Scientist Looks At Drugs (040 -80 BC) • Greek physician linked botany to this field & wrote materia medica, which included various drugs of vegetable origin. • He described various naturally occurring drugs & provided the basic information regarding its identification, cultivation, collection & storage.

Galen - Experimenter In Drug Compounding (131 -200 AD) Of the men of ancient times whose names are known and revered among both the professions of Pharmacy and Medicine, Galen, undoubtedly, is the foremost. Galen (130 -200 A. D. ) practiced and taught both Pharmacy and Medicine in Rome; His principles of preparing and compounding medicines ruled in the Western world for 1, 500 years; and his name still is associated with that class of pharmaceuticals compounded by mechanical means - galenicals. He was the originator of the formula for a cold cream, essentially similar to that known today. Many procedures Galen originated have their counterparts in today's modern compounding laboratories.

Paraselsus- Swiss physician (1493 -1541) • Responsible for developing the basic thinking of using individual chemical moiety for treatment of diseases.

Charak Samhita • Charak Samhita is one of the structuring dynamics of Rk Veda. • It highlights the BALANCING — HOLDING TOGETHER, NOURISHING, and SUPPORTING — quality involved in structuring Rk Veda. • With reference to consciousness, Charak Samhita comprises the specific sets of laws of Nature that are engaged in promoting the quality of Rishi — the observer, the witnessing quality — within the Samhita level of consciousness, providing a structure to the eternally silent, self-referral, self-sufficient, fully awake state of consciousness, which is intimately personal to everyone.

Origin, history & development of pharmacy contd. • Each government made some rules & the standards to maintain the uniformity & to control the standards of drugs & devices available in market. • Each country has its own book of standards which includes the list of the drugs along with related substances with their complete protocol. • These books are known as Pharmacopoeia

PHARMACOPOEIA • Pharmakon : a drug • Poieo: I make • Pharmacopoeia (literally, the art of the drug compounder), in its modern technical sense, is a book containing directions for the identification of samples and the preparation of compound medicines, and published by the authority of a government or a medical or pharmaceutical society.

Indian Pharmacopoeia (I. P. ) • Various editions of I. P. Edition Year 1 st Edition 1955 2 nd Edition 1966 3 rd Edition 1985 4 th Edition 1996 5 th Edition 2007

Indian Pharmacopoeia (I. P. ) • In 1944, Govt. of India asked the drugs Technical Advisory Board to prepare the list of drugs in use in India, having sufficient medicinal value to justify their inclusion in official pharmacopoeia. • Published by the Govt. of India under the name The Indian Pharmacopoeial List 1946 • The Committee constituted under the chairmanship of Lt. Col. Sir R. N. Chopra along with 9 members.

Indian Pharmacopoeia (I. P. ) • 1. The Indian Pharmacopoeial List 1946 Details Substances included in the British Pharmacopoeia, 48 monographs for crude drugs, chemicals & their preparations 2. Substances not included in British Pharmacopoeia: (a) (b) (c) (d) (e) (f) (g) (h) Drugs of Plant origin Drugs of animal origin Biological Products Insecticides Colouring agents Synthetics Miscellaneous Drugs for veterinary use 90 10 05 07 03 05 15 02

Indian Pharmacopoeia (I. P. ) 1955 • First edition published in 1955 • Written in English • The official Title of the monographs are given in Latin • Total 986 Monographs (includes crude-drugs, chemicals, biologicals & several formulae derived from them) • Supplements to first edition in 1960

Indian Pharmacopoeia (I. P. ) 1966 • 274 monographs from IP 55 & its supplement of 1960 were deleted • 93 new monographs were added • New analytical techniques were adapted • Usual strength for the preparations like tablets & injection was given.

Indian Pharmacopoeia (I. P. ) 1985 • Published in two Volumes • Nine appendices • 261 new monographs • 450 monographs included in 2 nd Edition were deleted • IUPAC nomenclature has been used • New Analytical Techniques • Dissolution test • Limit test for microbial contamination

Indian Pharmacopoeia (I. P. ) 1996 • Made effective from 1 st Dec 1996 • 1149 monographs • 123 appendices • 294 new monographs • 110 included in 3 rd edition were deleted • General monographs of formulations • Addendums of I. P. 1996 • Addendums 2000 • Addendums 2002

Indian Pharmacopoeia (I. P. ) 2007 • prepared by the Indian Pharmacopoeia Commission (IPC) in accordance with a plan and completed through the untiring efforts of its members and its Secretariat over a period of about two years. • This is the fifth edition of the Indian Pharmacopoeia after Independence. • It supersedes the 1996 edition but any monograph of the earlier edition that does not figure in this edition continues to be official as stipulated in the Second Schedule of the Drugs and Cosmetics Act, 1940.

Indian Pharmacopoeia (I. P. ) 2007 • The Indian Pharmacopoeia 2007 is presented in three volumes. • Volume 1 contains the Notice, Preface, the structure of the IPC, Acknowledgements, Introduction, and the General Chapters. • Volume 2 deals with the General Monographs on Drug Substances, Dosage Forms and Pharmaceutical Aids (A to M). • Volume 3 contains Monographs on Drug Substances, Dosage Forms and Pharmaceutical Aids (N to Z) followed by Monographs on Vaccines and Immunosera for Human use, Herbs and Herbal products, Blood and blood-related products, Biotechnology products and Veterinary products.

Indian Pharmacopoeia (I. P. ) 2007 • The scope of the Pharmacopoeia has been extended to include products of biotechnology, indigenous herbs and herbal products, viral vaccines and additional antiretroviral drugs and formulations, inclusive of commonly used fixeddose combinations. • Standards for veterinary drugs and products that were published as a Supplement to the previous edition of the Indian Pharmacopoeia now form an integral part of this compendium.

British Pharmacopoeia • London Pharmacopoeia first published in 1618 • British Pharmacopoeia published in 1864 through the merger of the London, Edinburgh and Dublin Pharmacopoeias • Legally enforced in UK through Medicines Act 1968 • National pharmacopoeias in EU enforced by Annex 1 of • Directive 2001/83/EC, the Community Code relating to medicinal products for human use • Published in UK by the British Pharmacopoeia Commission on behalf of UK Health Ministers • Legal Status in Australia and Canada

British Pharmacopoeia • Monographs for Chemical and pharmaceutical substances • General monographs for dosage forms • Monographs for finished dosage forms • One of only two English texts for monographs for finished dosage forms

British Pharmacopoeia • Published annually • Incorporate all the monographs of the current European Pharmacopoeia including its supplements • Provides the user with one comprehensively indexed compendium of all current UK pharmacopoeial standards • Links standards for pharmaceutical substances and formulated preparations • Includes reference infra-red spectra • Ph Eur monographs identified by a European chaplet of stores

United State Pharmacopoeia (USP) • The United States Pharmacopeia (USP) is the official public standards-setting authority for all prescription and over-the-counter medicines, dietary supplements, and other healthcare products manufactured and sold in the United States. • USP sets standards for the quality of these products and works with healthcare providers to help them reach the standards. • USP's standards are also recognized and used in more than 130 countries. • These standards have been helping to ensure good pharmaceutical care for people throughout the world for more than 185 years.

United State Pharmacopoeia (USP) • 1820 U. S. Pharmacopeia founded; all state societies of medicine invited to send delegates; 11 attended. USP created a system of standards, a system of quality control (formulae), and a national formulary. Only 217 drugs that met the criteria of "most fully established and best understood" were admitted. • 1830 Committee of Revision created (seven members); first revision of the USP published; revisions continue at 10 -year intervals. Surgeons General of U. S. Army and Navy became first federal agencies to participate in USP revision. • 1848 Drug Import Act–Federal legislation recognizes the USP as an official compendium. • 1850 Colleges of pharmacy invited to participate in revision of the USP. • 1880 s– 1890 s State Boards of Pharmacy formed; USP becomes a state board requirement. • 1900 USP incorporated in D. C. as not-for-profit corporation. U. S. Pharmacopeial Convention and Board of Trustees created. • 1906 Federal Food & Drugs Act: USP and NF strength, quality, and purity recognized as official standards.

United State Pharmacopoeia (USP) • 1938 Federal Food & Cosmetic Act: USP, NF, and Homeopathic Pharmacopeia standards of strength, quality, purity, packaging, and labeling recognized as official and enforced by FDA. "New Drug" concept established. FDA approves drugs for safety before marketing. • 1942 USP revision cycle changed; USP published every 5 years. • 1950 USP establishes its first permanent office in New York City. Lloyd C. Miller, Ph. D. , becomes first employed director of revision. • 1963 United States Adopted Names Council formed. Representatives from American Medical Association (AMA), American Pharmacists Association (APh. A), USP, and FDA establish drug nomenclature. • 1968 USP moves its headquarters from New York City, eventually locating in Rockville, Maryland, USA. • 1970 USP adopts resolution calling for information to be provided to dispensers of drugs in United States. Position of executive director created, filled by William M. Heller, Ph. D.

United State Pharmacopoeia (USP) • 1975 USP acquires National Formulary and Drug Standards Laboratory from APh. A. • 1977 USP and NF scope redefined: USP standards for drug substances and dosage forms; NF standards for excipients. • 1980 USP XX and the NF XV published under same cover; USP Dispensing Information, 1 st Edition. • 1990 Jerome A. Halperin becomes USP executive director (title later changed to executive vice president and CEO) at the 1990 USP Convention. USP members adopt a resolution to explore establishing standards for vitamins and minerals. • 1990, 1993 Federal Omnibus Budget Reconciliation Acts (OBRA '90 and '93) name the USP Drug Information as a source of information that state Medicaid agencies could use for drug utilization review, patient counseling, and medically accepted off-label uses of medicines. • 1994 USP signs an agreement with the American Medical Association to combine the information in AMA's Drug Evaluations database with the USP DI database to develop a single product that contains drug and therapeutic information.

United State Pharmacopoeia (USP) • 1995 USP members adopt a resolution to explore establishing standards for botanical dietary supplements. • 1997 The management consulting firm, Mc. Kinsey & Company, recommended new USP focus, including divestiture of print and electronic drug information products. • 1998 USP sells USP DI and associated products to The Thomson Company and USP DI is published by their MICROMEDEX subsidiary. The new Reference Standard Center opens. MEDMARX®, an Internet-accessible medication errors reporting program for hospitals, is launched. • 1999 USP publishes final USP DI in three-volume format; publishes USP 24–NF 19; initiated modernization project for developing e-commerce capabilities and establishing electronic communications with constituents. • 2000 At the USP Convention in April 2000, Roger L. Williams, M. D. , becomes USP executive vice president and CEO; members vote to change name of Committee of Revision to Council of Experts and elect chairpersons for 62 Expert Committees. • 2002 USP–NF published annually.

European Pharmacopoeia • The European Pharmacopoeia of the Council of Europe is a listing of a wide range of active substances and excipients used to prepare pharmaceutical products in Europe. • The European Pharmacopoeia is developed by the European Directorate for the Quality of Medicines (EDQM) and is a part of the Council of Europe, Strasbourg, France. • It bases on the Convention on the elaboration of a European Pharmacopoeia from 1964. • The 2005 edition includes 1800 specific and general monographs, including various chemical substances, antibiotics, biological substances; Vaccines for human or veterinary use; Immunosera; Radiopharmaceutical preparations; Herbal drugs; Homoeopathic preparations and homoeopathic stocks. • It also contains Dosage forms, General monographs, Materials and Containers, Sutures; 268 General methods with figures or chromatograms and 2210 reagents are described. • The monographs give quality standards for all the main medicines used in Europe.

European Pharmacopoeia • 1 st edition - published 1967 • 2 nd edition - published 1980 • 3 rd edition - published 1997 • 4 th edition - published 2001, valid from 1. 1. 2002 • 5 th edition - published 15. 6. 2004, valid from 1. 1. 2005 • 6 th edition - published 16 July 2007, valid from 1. 1. 2008 • It is published by EDQM in English and French, official national translations are available in German and Spanish (Spanish version only online)
 Selection and gradation in language teaching
Selection and gradation in language teaching Historical perspective of community development
Historical perspective of community development Greek theatre history
Greek theatre history Psychology definition
Psychology definition Explain methods of psychology
Explain methods of psychology Structuralist school of thought
Structuralist school of thought Definition and scope of pharmacy
Definition and scope of pharmacy Stanislas limousin contribution
Stanislas limousin contribution Empiric era of pharmacy
Empiric era of pharmacy History of pharmacy informatics
History of pharmacy informatics History of pharmacy informatics
History of pharmacy informatics Evolution of pharmacy
Evolution of pharmacy Development that ended much development crossword
Development that ended much development crossword Truncated cylinder development
Truncated cylinder development Lec hardver
Lec hardver History
History History of development communication ppt
History of development communication ppt History and development of computer
History and development of computer History of career guidance in the philippines
History of career guidance in the philippines Brief history of multimedia
Brief history of multimedia History and development of accounting
History and development of accounting Android development history
Android development history History of rapid application development
History of rapid application development History also history physical
History also history physical Daoism founder
Daoism founder Whip-cracking in the czech republic and slovakia
Whip-cracking in the czech republic and slovakia Same origin policy example
Same origin policy example Vestibulospinal tract origin
Vestibulospinal tract origin Kinocillium
Kinocillium Vertigo causes
Vertigo causes Origin myths definition
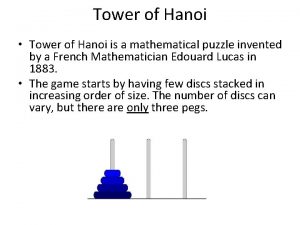
Origin myths definition Tower of hanoi origin
Tower of hanoi origin Certificate of origin
Certificate of origin