Heavy metal toxicity Overview There are 35 metals











- Slides: 12

Heavy metal toxicity

Overview • There are 35 metals that concern us because of occupational or residential exposure. • 23 of these are the heavy elements or "heavy metals": antimony, arsenic, bismuth, cadmium, cerium, chromium, cobalt, copper, gallium, gold, iron, lead, manganese, mercury, nickel, platinum, silver, tellurium, thallium, tin, uranium, vanadium, and zinc. • Heavy metals" are chemical elements with a specific gravity that is at least 5 times the specific gravity of water. The specific gravity of water is 1 at 4°C (39°F).

Beneficial and toxic heavy metals • In small quantities, certain heavy metals are nutritionally essential for a healthy life. Some of these are referred to as the trace elements (e. g. , iron, copper, manganese, and zinc). These elements, or some form of them, are commonly found naturally in foodstuffs. • Heavy metals become toxic when they are not metabolized by the body and accumulate in the soft tissues. Heavy metals may enter the human body through food, water, air, or absorption through the skin when they come in contact with humans in agriculture and in manufacturing, pharmaceutical, industrial, or residential settings.

Pathophysiology • The pathophysiology of the heavy metal toxidromes remains relatively constant. For the most part, heavy metals bind to oxygen, nitrogen, and sulfhydryl groups in proteins, resulting in alterations of enzymatic activity. • Although ligand formation is the basis for much of the transport of heavy metals throughout the body, some metals may compete with ionized species such as calcium and zinc to move through membrane channels in the free ionic form. • For example, lead follows calcium pathways in the body, hence its deposition in bone and gingivae. Thallium is taken up into cells like potassium because of their similar ionic radii. • Nearly all organ systems are involved in heavy metal toxicity; however, the most commonly involved organ systems include the CNS, PNS, GI, hematopoietic, renal, and cardiovascular (CV). • To a lesser extent, lead toxicity involves the musculoskeletal and reproductive systems. The organ systems affected and the severity of the toxicity vary with the particular heavy metal involved, the chronicity and extent of the exposure, and the age of the individual.

CHELATING AGENTS, HERBICIDES AND INSECTICIDES Dr A. Shyam Sundar, Assistant Professor in Phrmacology, University of Nizwa, Sultanate of Oman

CHELATING AGENTS, HERBICIDES AND INSECTICIDES Expected Lecture Outcomes • Understand the applications on chelating agents in a clinical setup. • Respond to the threat of intoxication of herbicides and insecticides. • Acquire knowledge about the various means of possible exposure. • Understand an overview of protocol for managing toxic ingestions, and the antidotes and treatments associated with their pathology.

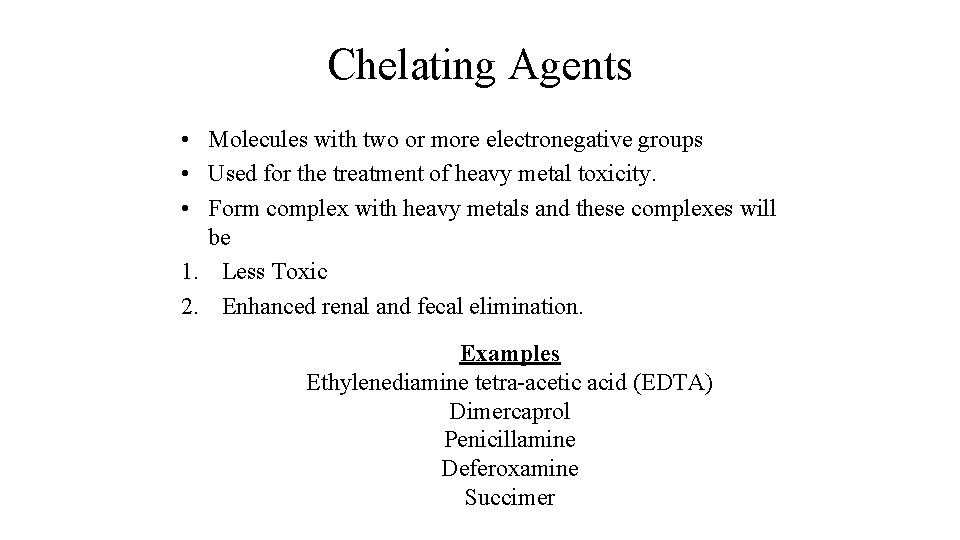
Chelating Agents • Molecules with two or more electronegative groups • Used for the treatment of heavy metal toxicity. • Form complex with heavy metals and these complexes will be 1. Less Toxic 2. Enhanced renal and fecal elimination. Examples Ethylenediamine tetra-acetic acid (EDTA) Dimercaprol Penicillamine Deferoxamine Succimer

EDTA • • Primarily used to treat lead poisoning Administered parenterally (i. v or i. m) Most important toxicity is nephrotoxicity (reversible) Sodium salt of EDTA causes severe hypocalcaemia, so calcium disodium salt of EDTA is used clinically.

Dimercaprol(BAL) • First developed as an antidote for lewisite(Arsenical war gas) • Used in the treatment of mercury, arsenic, lead and cadmium toxicities • Administered intra-muscularly • Contraindicated in: 1. 2. 3. Concurrent iron therapy G-6 -PD deficiency Peanut oil allergy

Penicillamine • Used in copper poisoning (Wilson’s Disease), lead, arsenic, mercury and gold toxicities. Used in Rheumatoid Arthritis and Cystinuria. • Administered enterally (only commercially available oral chelating agent for adults). • Adverse Effects: Rash, Fever, Leucopenia and Thrombocytopenia (common) Aplastic Anemia, SJ Syndrome and Lupus erythematosus (rare)

Deferoxamine • Used for acute iron( ferrous salts) poisoning. • Administered parenterally • Poorly competes with the ‘heme’ part of hemoglobin and cytochromes. • Adverse Effects: 1. Neurotoxicity 2. 3. 4. Hepatic and renal dysfunction Severe Coagulopathies Hypotensive Shock when given through i. v, due to histamine release.

Succimer • Oral congener of dimercaprol • Lead intoxication of kids (serum level>45 mcg/dl • Adverse Effects: 1. 2. 3. Transient increase in hepatic transaminases. Skin rash GI distress
 Insidan region jh
Insidan region jh What does ratey stand for
What does ratey stand for Ferrous material
Ferrous material Examples of metals
Examples of metals Characteristics of metals
Characteristics of metals What is matter in natural science
What is matter in natural science Metals and non metals in the periodic table
Metals and non metals in the periodic table Natural science grade 7 matter and materials
Natural science grade 7 matter and materials One way ticket to midnight heavy metal
One way ticket to midnight heavy metal Heavy metal pollution
Heavy metal pollution State of matter venn diagram
State of matter venn diagram Liquid elements at room temperature
Liquid elements at room temperature Non metals and uses
Non metals and uses






















