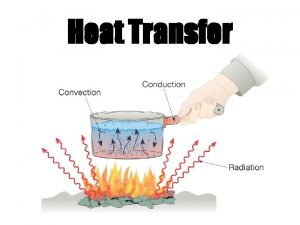
Heat Transfer greenmoxie com Transfer Mechanisms Heat can

![Transfer Mechanisms ] Heat can be transferred in three ways. Transfer can include more Transfer Mechanisms ] Heat can be transferred in three ways. Transfer can include more](https://slidetodoc.com/presentation_image_h2/767afc94f7b9702f3e9a769ac450ec11/image-2.jpg)
![Direct Contact ] Items in direct contact transfer heat. ] Molecules in hot regions Direct Contact ] Items in direct contact transfer heat. ] Molecules in hot regions](https://slidetodoc.com/presentation_image_h2/767afc94f7b9702f3e9a769ac450ec11/image-3.jpg)
![Thermal Conductivity ] Heat flow within an object is due to transfer by conduction. Thermal Conductivity ] Heat flow within an object is due to transfer by conduction.](https://slidetodoc.com/presentation_image_h2/767afc94f7b9702f3e9a769ac450ec11/image-4.jpg)
![Conductors and Insulators ] Thermal conductors have high values of k. • Metals with Conductors and Insulators ] Thermal conductors have high values of k. • Metals with](https://slidetodoc.com/presentation_image_h2/767afc94f7b9702f3e9a769ac450ec11/image-5.jpg)
![Thermal Resistance ] The rate of heat flow depends on the temperature gradient. • Thermal Resistance ] The rate of heat flow depends on the temperature gradient. •](https://slidetodoc.com/presentation_image_h2/767afc94f7b9702f3e9a769ac450ec11/image-6.jpg)
![Material Flow ] Objects at different temperatures may not be in direct thermal contact. Material Flow ] Objects at different temperatures may not be in direct thermal contact.](https://slidetodoc.com/presentation_image_h2/767afc94f7b9702f3e9a769ac450ec11/image-7.jpg)
![Gay-Lussac’s Law ] Pressure is also related to the temperature. ] The effect is Gay-Lussac’s Law ] Pressure is also related to the temperature. ] The effect is](https://slidetodoc.com/presentation_image_h2/767afc94f7b9702f3e9a769ac450ec11/image-8.jpg)
![Fluid Motion ] Objects in a fluid have a force of buoyancy. ] Materials Fluid Motion ] Objects in a fluid have a force of buoyancy. ] Materials](https://slidetodoc.com/presentation_image_h2/767afc94f7b9702f3e9a769ac450ec11/image-9.jpg)
![Circulation ] If one area in a fluid is heated that are will become Circulation ] If one area in a fluid is heated that are will become](https://slidetodoc.com/presentation_image_h2/767afc94f7b9702f3e9a769ac450ec11/image-10.jpg)
![Home Heating ] Convection causes heat to circulate in a house. • Water circulates Home Heating ] Convection causes heat to circulate in a house. • Water circulates](https://slidetodoc.com/presentation_image_h2/767afc94f7b9702f3e9a769ac450ec11/image-11.jpg)
![Global Circulation ] Warm air at the equator also rises. ] As it moves Global Circulation ] Warm air at the equator also rises. ] As it moves](https://slidetodoc.com/presentation_image_h2/767afc94f7b9702f3e9a769ac450ec11/image-13.jpg)
![Hot Metal ] When metal is heated enough it glows. ] The color changes Hot Metal ] When metal is heated enough it glows. ] The color changes](https://slidetodoc.com/presentation_image_h2/767afc94f7b9702f3e9a769ac450ec11/image-14.jpg)
![Radiation from Heat ] Heated objects give off electromagnetic waves. • Higher temperature has Radiation from Heat ] Heated objects give off electromagnetic waves. • Higher temperature has](https://slidetodoc.com/presentation_image_h2/767afc94f7b9702f3e9a769ac450ec11/image-15.jpg)
![Blackbody ] ] ] Objects can both absorb and emit radiation. Good absorbers are Blackbody ] ] ] Objects can both absorb and emit radiation. Good absorbers are](https://slidetodoc.com/presentation_image_h2/767afc94f7b9702f3e9a769ac450ec11/image-16.jpg)
![Seasons ] Earth absorbs radiation from the Sun. ] The tilt of Earth’s axis Seasons ] Earth absorbs radiation from the Sun. ] The tilt of Earth’s axis](https://slidetodoc.com/presentation_image_h2/767afc94f7b9702f3e9a769ac450ec11/image-17.jpg)
![Greenhouse Effect ] ] The atmosphere only permits visible light and radio waves. Visible Greenhouse Effect ] ] The atmosphere only permits visible light and radio waves. Visible](https://slidetodoc.com/presentation_image_h2/767afc94f7b9702f3e9a769ac450ec11/image-18.jpg)
![Ozone ] High frequency UV light forms ozone from oxygen. • Stratosphere, mesophere ] Ozone ] High frequency UV light forms ozone from oxygen. • Stratosphere, mesophere ]](https://slidetodoc.com/presentation_image_h2/767afc94f7b9702f3e9a769ac450ec11/image-19.jpg)
- Slides: 19

Heat Transfer greenmoxie. com
![Transfer Mechanisms Heat can be transferred in three ways Transfer can include more Transfer Mechanisms ] Heat can be transferred in three ways. Transfer can include more](https://slidetodoc.com/presentation_image_h2/767afc94f7b9702f3e9a769ac450ec11/image-2.jpg)
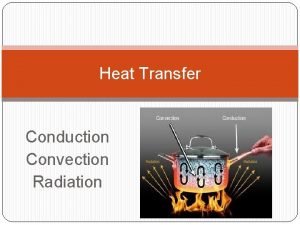
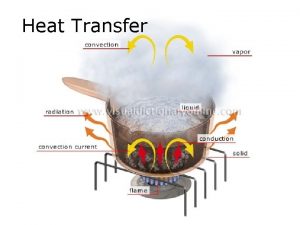
Transfer Mechanisms ] Heat can be transferred in three ways. Transfer can include more than one way. ] Conduction ] • Heat from direct thermal contact ] Convection • Fluid flow carrying energy ] Radiation • Energy radiating from an object into surroundings
![Direct Contact Items in direct contact transfer heat Molecules in hot regions Direct Contact ] Items in direct contact transfer heat. ] Molecules in hot regions](https://slidetodoc.com/presentation_image_h2/767afc94f7b9702f3e9a769ac450ec11/image-3.jpg)
Direct Contact ] Items in direct contact transfer heat. ] Molecules in hot regions have greater kinetic energy. • Elastic collisions with cool molecules • Kinetic energy transfer at boundary
![Thermal Conductivity Heat flow within an object is due to transfer by conduction Thermal Conductivity ] Heat flow within an object is due to transfer by conduction.](https://slidetodoc.com/presentation_image_h2/767afc94f7b9702f3e9a769ac450ec11/image-4.jpg)
Thermal Conductivity ] Heat flow within an object is due to transfer by conduction. ] Thermal conductivity (k) measures the ability for heat to move in a material. • Measured in W / m-K • High number means high rate of transfer Material Air Stryrofoam Wood Water Glass Concrete Steel Aluminum Copper Thermal Cond. 0. 026 W/m-K 0. 029 W/m-K 0. 11 W/m-K 0. 61 W/m-K 0. 8 W/m-K 1. 0 W/m-K 46 W/m-K 240 W/m-K 400 W/m-K
![Conductors and Insulators Thermal conductors have high values of k Metals with Conductors and Insulators ] Thermal conductors have high values of k. • Metals with](https://slidetodoc.com/presentation_image_h2/767afc94f7b9702f3e9a769ac450ec11/image-5.jpg)
Conductors and Insulators ] Thermal conductors have high values of k. • Metals with conducting electrons • Greater than 10 W/m-K ] Still air is an excellent thermal insulator. • Materials that trap air are good: wood, styrofoam ] Vacuum would be the best.
![Thermal Resistance The rate of heat flow depends on the temperature gradient Thermal Resistance ] The rate of heat flow depends on the temperature gradient. •](https://slidetodoc.com/presentation_image_h2/767afc94f7b9702f3e9a769ac450ec11/image-6.jpg)
Thermal Resistance ] The rate of heat flow depends on the temperature gradient. • Change in temperature with distance A H T + DT T Dx ] In the US, thermal resistance is measured per unit area. • R = Dx / k • Units are ft 2 F hr / BTU • 1 BTU = 1055 J Material R-factor Glass (1/8”) 1 Brick (3½”) 0. 6 – 1 Plywood (1/2”) 0. 6 Fiberglass insulation (1”) 4
![Material Flow Objects at different temperatures may not be in direct thermal contact Material Flow ] Objects at different temperatures may not be in direct thermal contact.](https://slidetodoc.com/presentation_image_h2/767afc94f7b9702f3e9a769ac450ec11/image-7.jpg)
Material Flow ] Objects at different temperatures may not be in direct thermal contact. ] An intermediate mechanism is needed to transfer heat. ] If the heat transfer is through the motion of a fluid, then it is called convection.
![GayLussacs Law Pressure is also related to the temperature The effect is Gay-Lussac’s Law ] Pressure is also related to the temperature. ] The effect is](https://slidetodoc.com/presentation_image_h2/767afc94f7b9702f3e9a769ac450ec11/image-8.jpg)
Gay-Lussac’s Law ] Pressure is also related to the temperature. ] The effect is linear if the volume is kept the same. ] Named after Joseph Gay. Lussac who observed it in the early 19 th century. hot cold high P low P
![Fluid Motion Objects in a fluid have a force of buoyancy Materials Fluid Motion ] Objects in a fluid have a force of buoyancy. ] Materials](https://slidetodoc.com/presentation_image_h2/767afc94f7b9702f3e9a769ac450ec11/image-9.jpg)
Fluid Motion ] Objects in a fluid have a force of buoyancy. ] Materials expand when they are heated. • Increased volume • Lower density ] Warm fluids generally rise. Fb = mg
![Circulation If one area in a fluid is heated that are will become Circulation ] If one area in a fluid is heated that are will become](https://slidetodoc.com/presentation_image_h2/767afc94f7b9702f3e9a769ac450ec11/image-10.jpg)
Circulation ] If one area in a fluid is heated that are will become less dense. ] As it moves away it transfers energy and cools ] Cool fluid replaces the warm fluid that left.
![Home Heating Convection causes heat to circulate in a house Water circulates Home Heating ] Convection causes heat to circulate in a house. • Water circulates](https://slidetodoc.com/presentation_image_h2/767afc94f7b9702f3e9a769ac450ec11/image-11.jpg)
Home Heating ] Convection causes heat to circulate in a house. • Water circulates in pipes • Air circulates around radiators. ] Forced circulation uses a fan to push heated air. • Faster exchange of heat

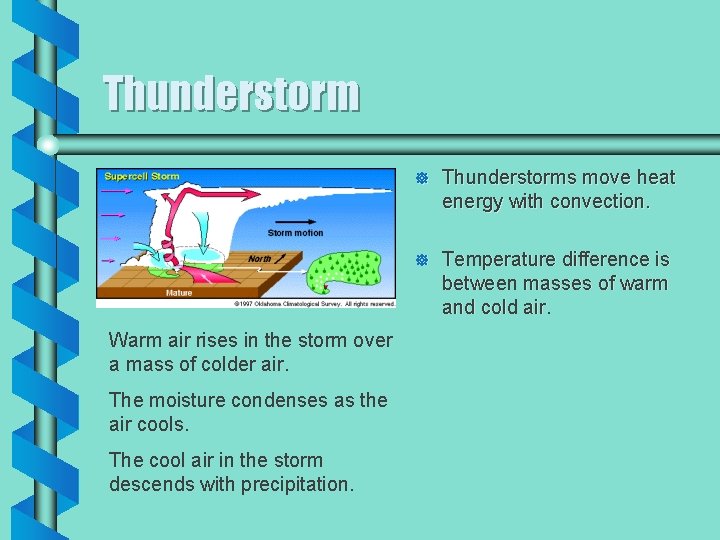
Thunderstorm Warm air rises in the storm over a mass of colder air. The moisture condenses as the air cools. The cool air in the storm descends with precipitation. ] Thunderstorms move heat energy with convection. ] Temperature difference is between masses of warm and cold air.
![Global Circulation Warm air at the equator also rises As it moves Global Circulation ] Warm air at the equator also rises. ] As it moves](https://slidetodoc.com/presentation_image_h2/767afc94f7b9702f3e9a769ac450ec11/image-13.jpg)
Global Circulation ] Warm air at the equator also rises. ] As it moves poleward it cools. ] The cool air descends. ] This forms a Hadley cell.
![Hot Metal When metal is heated enough it glows The color changes Hot Metal ] When metal is heated enough it glows. ] The color changes](https://slidetodoc.com/presentation_image_h2/767afc94f7b9702f3e9a769ac450ec11/image-14.jpg)
Hot Metal ] When metal is heated enough it glows. ] The color changes with temperature. • Red – orange – yellow – white ] Radiation can cross a vacuum and carry energy.
![Radiation from Heat Heated objects give off electromagnetic waves Higher temperature has Radiation from Heat ] Heated objects give off electromagnetic waves. • Higher temperature has](https://slidetodoc.com/presentation_image_h2/767afc94f7b9702f3e9a769ac450ec11/image-15.jpg)
Radiation from Heat ] Heated objects give off electromagnetic waves. • Higher temperature has more radiation ] intensity low energy high energy A hot object gives off a spectrum of frequencies. • Shifts based on temperature frequency
![Blackbody Objects can both absorb and emit radiation Good absorbers are Blackbody ] ] ] Objects can both absorb and emit radiation. Good absorbers are](https://slidetodoc.com/presentation_image_h2/767afc94f7b9702f3e9a769ac450ec11/image-16.jpg)
Blackbody ] ] ] Objects can both absorb and emit radiation. Good absorbers are also good emitters. Emissivity measures the ability of an object to radiate heat. • A perfect emissivity of 1 is a blackbody. Low: near 0 High: near 1
![Seasons Earth absorbs radiation from the Sun The tilt of Earths axis Seasons ] Earth absorbs radiation from the Sun. ] The tilt of Earth’s axis](https://slidetodoc.com/presentation_image_h2/767afc94f7b9702f3e9a769ac450ec11/image-17.jpg)
Seasons ] Earth absorbs radiation from the Sun. ] The tilt of Earth’s axis changes the radiation absorbed. • Summer vs winter
![Greenhouse Effect The atmosphere only permits visible light and radio waves Visible Greenhouse Effect ] ] The atmosphere only permits visible light and radio waves. Visible](https://slidetodoc.com/presentation_image_h2/767afc94f7b9702f3e9a769ac450ec11/image-18.jpg)
Greenhouse Effect ] ] The atmosphere only permits visible light and radio waves. Visible light warms the surface. The surface absorbs visible light and radiates infrared. The infrared is trapped and warms the air. visible light atmosphere infrared light earth
![Ozone High frequency UV light forms ozone from oxygen Stratosphere mesophere Ozone ] High frequency UV light forms ozone from oxygen. • Stratosphere, mesophere ]](https://slidetodoc.com/presentation_image_h2/767afc94f7b9702f3e9a769ac450ec11/image-19.jpg)
Ozone ] High frequency UV light forms ozone from oxygen. • Stratosphere, mesophere ] Ozone concentrates in a layer in the stratosphere. • Opaque to UV-B • Dissolved by CFCs NASA
 Bài thơ mẹ đi làm từ sáng sớm
Bài thơ mẹ đi làm từ sáng sớm Cơm
Cơm Heat transfer objectives
Heat transfer objectives Risk transfer mechanisms
Risk transfer mechanisms Technology transfer mechanisms
Technology transfer mechanisms Thermoneutral environment for neonates
Thermoneutral environment for neonates Mechanisms of heat loss in newborn
Mechanisms of heat loss in newborn Repeating disturbance that transfers energy
Repeating disturbance that transfers energy Mechanisms of movement lab report
Mechanisms of movement lab report Virtualization ppt
Virtualization ppt Mischel's theory
Mischel's theory What animals are cold blooded
What animals are cold blooded Projection example defense mechanism
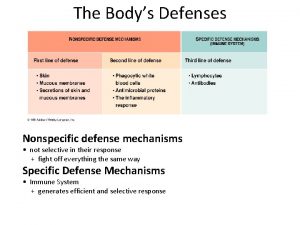
Projection example defense mechanism What are the first line of defense
What are the first line of defense Four defense mechanisms
Four defense mechanisms Circular motion examples
Circular motion examples How does a sponge defend itself
How does a sponge defend itself Multi device broker in cloud computing
Multi device broker in cloud computing Secure attachment style
Secure attachment style Different types of pop up mechanisms
Different types of pop up mechanisms