Health Care Reform Spending Cuts and Drug Approvals


























- Slides: 37

Health Care Reform, Spending Cuts and Drug Approvals: What to Expect From the Obama Administration’s Second Term. : Kate Rawson. : Prevision Policy LLC. : January 9, 2012 Copyright © 2013 Prevision. Policy. All rights reserved.

Credit Where Credit is Due The Prevision Policy Team Cole Werble, Founding Member Michael Mc. Caughan, Founding Member Ramsey Baghdadi, Founding Member Kate Rawson, Senior Editor Laura Helbling, Reporter/Analyst Material from this presentation is derived from interviews with company executives and investors, and from transaction data tracked by Windhover Information Inc. , publisher of The RPM Report. Copyright © 2013 Prevision. Policy. All rights reserved.

Agenda “Status Quo” Election Major agency heads expected to remain in office Congressional changes due to retirements and primary defeats Affordable Care Act Survives (Barely) Ruling is generally a positive for biopharma companies SCOTUS ruling on Medicaid expansion is one downside; IPAB impact is worrisome to industry FDA Innovation & Safety Act New incentives for drug development in unmet areas (antibiotics, rare disease, “Breakthrough”) “Fixes” drug shortage problem Provisions to increase manufacturing quality of facilities overseas FDA Drug Approvals: It Was a Very Good Year “The Program”: focus on novel drugs with more meetings, greater transparency Review times lengthen for novel drug and biologics Will it improve first-cycle review percentage? Hard to improve on near-perfection Congress Avoids the Cliff: What Does That Mean for Rx Risk? Usual suspects could be on the chopping block Series of smaller cuts vs. a “Grand Bargain” Big question is whether pharma can protect Part D rebates for dual-eligibles Copyright © 2013 Prevision. Policy. All rights reserved.

Life After 2012 The Year of the Patent Cliff Lipitor, Plavix, Zyprexa, etc A Wild Year in Washington PDUFA V/FDASIA Supreme Court ACA Review Election Day Caps a Disastrous Decade Pipeline Drought Death of the Blockbuster Corporate Downsizing BUT THERE IS HOPE! 4 Copyright © 2013 Prevision. Policy. All rights reserved.

A “Status Quo” Election… Copyright © 2013 Prevision. Policy. All rights reserved.

…Means “Status Quo” Agency Leadership Copyright © 2013 Prevision. Policy. All rights reserved.

Affordable Care Act Survives (Barely) "It is not our job to save the people from the consequences of their electoral choices. ” Copyright © 2013 Prevision. Policy. All rights reserved.

Affordable Care Act Survives (Barely) • Ruling is Positive For Biopharma; Robust Growth Expected… • CMS projects national drug spending be 8. 8% in 2014 (versus 2. 4% in 2013) • 4. 7 percentage points faster than in the absence of health care reform • Medicaid enrollment to increase by up to 19. 6 million people in 2014 • Retains IP protections contained in the biosimilars provisions in the ACA • …But There Are Potential Downsides • SCOTUS decision allows states to opt out of Medicaid expansion risk-free • Impact of Independent Payment Advisory Board (IPAB) yet to be seen Copyright © 2013 Prevision. Policy. All rights reserved.

2013: A Year of Implementation Affordable Care Act Oct. 1 Deadline to Launch Exchanges Medicaid Expansion Still Fluid Final Regs Coming Soon: • “Sunshine” Act on Provider/Pharma Payments • Medicaid Rebate Rule FDA Safety & Innovation Act • Incentives for Antibiotics • Biosimilar Pathway Taking Shape, SLOWLY • Major Resources for Generic Drug Reviews • Supply Chain Impact • Foreign Inspections • Shortage Response • Compounding Copyright © 2013 Prevision. Policy. All rights reserved.

Innovation In FDASIA “Breakthrough Therapies” Hyper-Fast Development for Drugs That Show Extraordinary Clinical Efficacy Vertex has two: ivacaftor and ivacaftor/VX-809 combo for CF “Qualified” Infectious Disease Agents (GAIN Act) Five-Year Added Exclusivity For Anti-Infectives/Anti-Fungals Targeting Priority Infections “Enhanced” Accelerated Approval Codifies AA, Directs FDA to Update Guidance/Regs Does NOT Modify Approval Standard Rare Disease Provisions Pediatric Rare Disease Voucher Pediatric Exclusivity Made Permanent 10 Single-Enantiomer Provision Extended Five Years Copyright © 2013 Prevision. Policy. All rights reserved.

Implications of “Breakthrough” Therapies “What I think FDASIA does more than had been done previously is it says to all of FDA, management and staff, ‘Get into this stuff. ’… “If there’s a process for identifying things that are so impressive-looking that you’re going to designate them as breakthrough, the involvement should be not just at the division level, but broader than that. You’re going to have Jenkins in the room and maybe Janet. We should all be paying attention to how we do that. ” Bob Temple, FDA/CMS Summit December 11 Copyright © 2013 Prevision. Policy. All rights reserved.

GAIN Act: Anti-Infective Incentives “There's no area that we're working on harder than trying to develop pathways that are feasible and scientifically rigorous for developing and approving new antimicrobials. It is a public health crisis. We need new antimicrobials. ” -- John Jenkins, FDA/CMS Summit December 10 Copyright © 2013 Prevision. Policy. All rights reserved.

The “Lake Wobegon Effect” There are now at least 16 (!) “Special” designations at FDA: Where All The Drugs Are Above Average Copyright © 2013 Prevision. Policy. All rights reserved.

Drug Shortages: Unprecedented Attention FDASIA “Fixes” The Shortage Problem Copyright © 2013 Prevision. Policy. All rights reserved.

Congress Has Now “Fixed” This Problem… Even If No One Knows How… What Is In: • More Mandatory Reporting by Pharma • Non-Compliance Publicized, not Punished • “Flexibility” For Recovering Manufacturers • FDA Task Force • Inter-Agency Coordination • Input from Stakeholders, including 3 d party Reporting • Limited Hospital Re-Packaging • Intra-System Use Only What Is Not: • Change ASP/AMP Formulas • Incentivize Quality/Reliability of Supply • Medicaid Rebate/340 B “Holiday” • All Subject to Further Study • GAO Report Due in 18 Months Ideas “On The Shelf” For Next Time…. Copyright © 2013 Prevision. Policy. All rights reserved.

FDASIA: Supply Chain Elements “Upstream” Provisions Included Manufacturing Registration, Foreign Inspection, Import Controls “Good Import Practices” to Match GMP, GCP Model “Downstream” Deferred New Anti-Counterfeiting Provisions Included Pedigree, Track-and-Trace Are Not Track and Trace Discussions Continue; Draft Legislation Released For Public Comment Copyright © 2013 Prevision. Policy. All rights reserved.

Compounding The Issue… Rx Compounding Legislation Likely in 2013 May Be Vehicle for Additional Measures on Supply Chain Copyright © 2013 Prevision. Policy. All rights reserved.

PDUFA: Perpetual FDA Reform Routine Maintenance for FDA Congress Hauls in FDA Every 5 Years and Looks Under the Hood Copyright © 2013 Prevision. Policy. All rights reserved.

FDASIA and PDUFA V: Two Key Texts FDASIA “The Law” PDUFA V The “Goal Letter” Copyright © 2013 Prevision. Policy. All rights reserved.

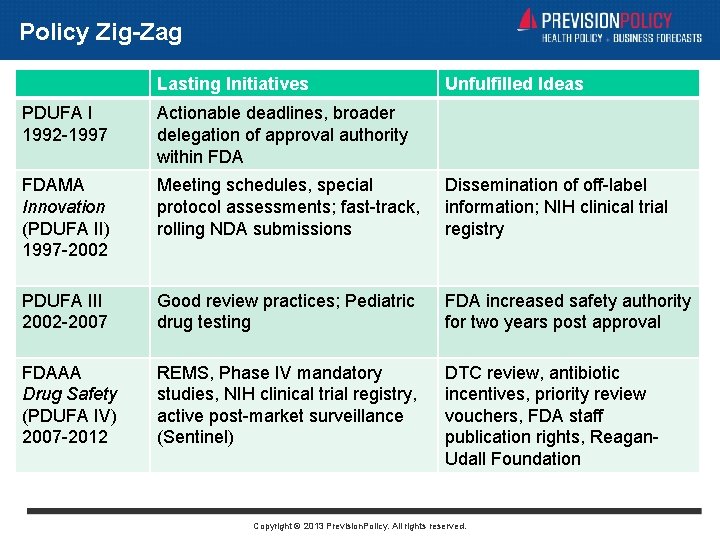
Policy Zig-Zag Lasting Initiatives Unfulfilled Ideas PDUFA I 1992 -1997 Actionable deadlines, broader delegation of approval authority within FDAMA Innovation (PDUFA II) 1997 -2002 Meeting schedules, special protocol assessments; fast-track, rolling NDA submissions Dissemination of off-label information; NIH clinical trial registry PDUFA III 2002 -2007 Good review practices; Pediatric drug testing FDA increased safety authority for two years post approval FDAAA Drug Safety (PDUFA IV) 2007 -2012 REMS, Phase IV mandatory studies, NIH clinical trial registry, active post-market surveillance (Sentinel) DTC review, antibiotic incentives, priority review vouchers, FDA staff publication rights, Reagan. Udall Foundation Copyright © 2013 Prevision. Policy. All rights reserved.

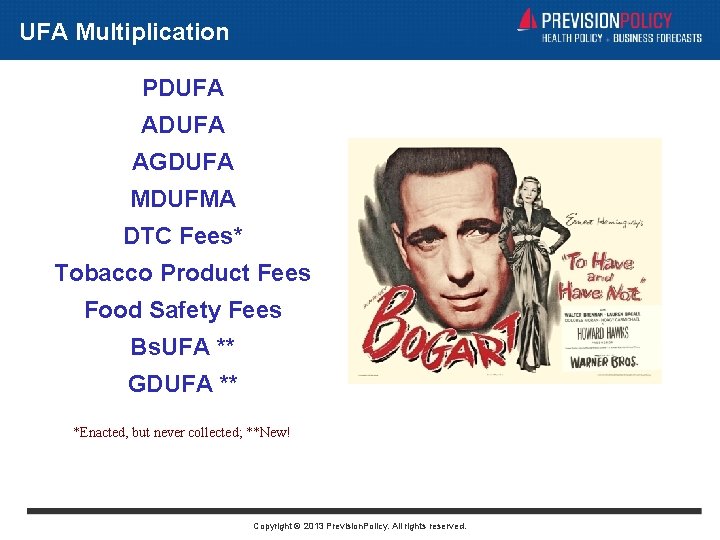
UFA Multiplication PDUFA AGDUFA MDUFMA DTC Fees* Tobacco Product Fees Food Safety Fees Bs. UFA ** GDUFA ** *Enacted, but never collected; **New! Copyright © 2013 Prevision. Policy. All rights reserved.

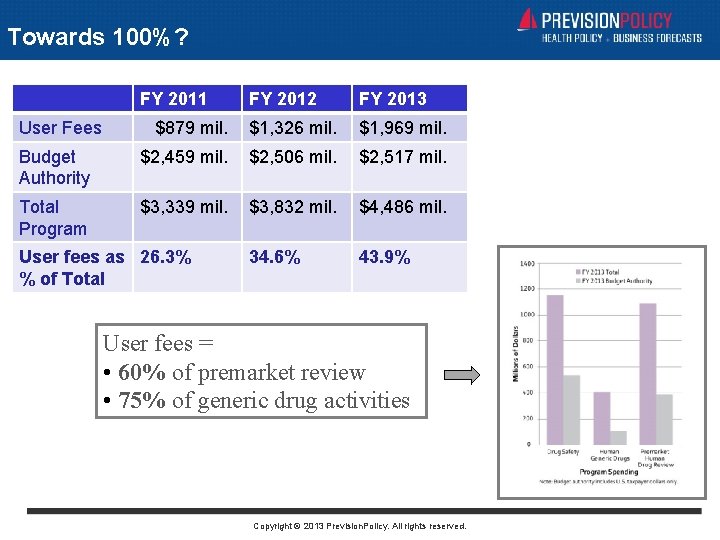
Towards 100%? FY 2011 FY 2012 FY 2013 $879 mil. $1, 326 mil. $1, 969 mil. Budget Authority $2, 459 mil. $2, 506 mil. $2, 517 mil. Total Program $3, 339 mil. $3, 832 mil. $4, 486 mil. 34. 6% 43. 9% User Fees User fees as 26. 3% % of Total User fees = • 60% of premarket review • 75% of generic drug activities Copyright © 2013 Prevision. Policy. All rights reserved.

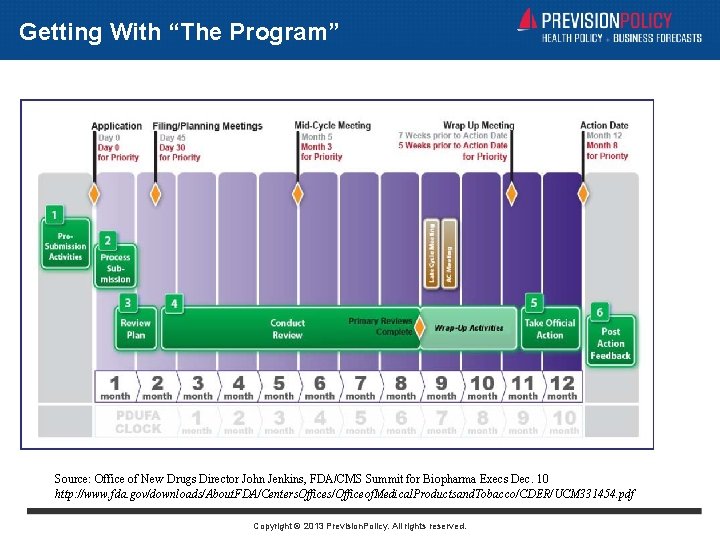
PDUFA V: Getting With “The Program” • Applies to NMEs Only • Two Month LONGER Reviews (12 or 8 months) • First Time in PDUFA Era that Slower is Better • Buys Time for Inspections, REMS Negotiations • Pre-NDA Meeting is Vital • Though Technically Optional • Limited Opportunity to Supplement Filing • Expect RTFs for Incomplete Applications • Greater Emphasis on Planning Review • Multiple Check-Ins With Sponsor • Late-Cycle Meeting is Key Innovation (and Already Controversial) • Sponsors Want “Verbal Complete Response” • FDA Management Emphasizing Process Review • Decision Maker Engaged Copyright © 2013 Prevision. Policy. All rights reserved.

Getting With “The Program” Source: Office of New Drugs Director John Jenkins, FDA/CMS Summit for Biopharma Execs Dec. 10 http: //www. fda. gov/downloads/About. FDA/Centers. Offices/Officeof. Medical. Productsand. Tobacco/CDER/UCM 331454. pdf Copyright © 2013 Prevision. Policy. All rights reserved.

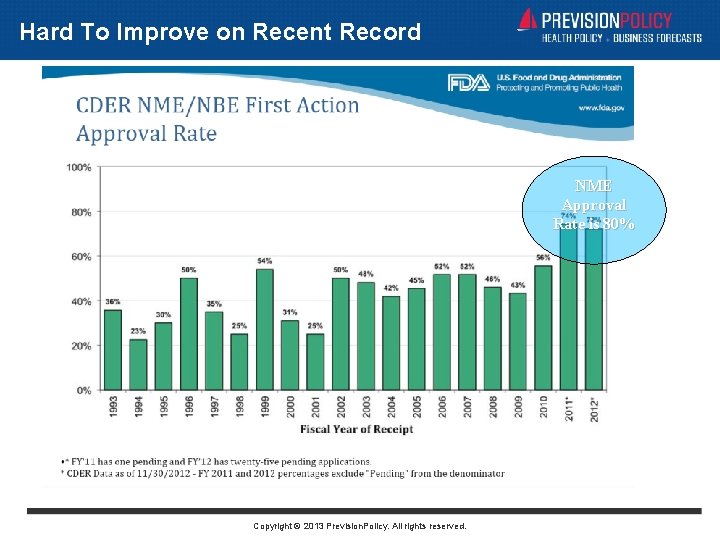
Getting With “The Program”: Implications • Better Meetings: • Outside Consultants in the Room • Fewer, Less Exciting Advisory Committees: • Late-Cycle Meeting Eliminates “Surprises” • New Communication Challenge for Sponsors: • What to Say About FDA Updates? • Unlikely to Improve “First-Cycle” Performance: • Tough To Beat Near Perfection Copyright © 2013 Prevision. Policy. All rights reserved.

Hard To Improve on Recent Record NME Approval Rate is 80% Copyright © 2013 Prevision. Policy. All rights reserved.

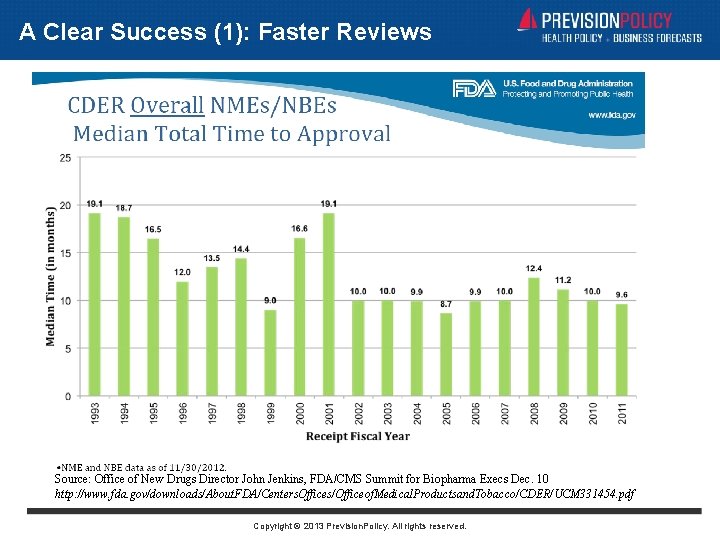
A Clear Success (1): Faster Reviews Source: Office of New Drugs Director John Jenkins, FDA/CMS Summit for Biopharma Execs Dec. 10 http: //www. fda. gov/downloads/About. FDA/Centers. Offices/Officeof. Medical. Productsand. Tobacco/CDER/UCM 331454. pdf Copyright © 2013 Prevision. Policy. All rights reserved.

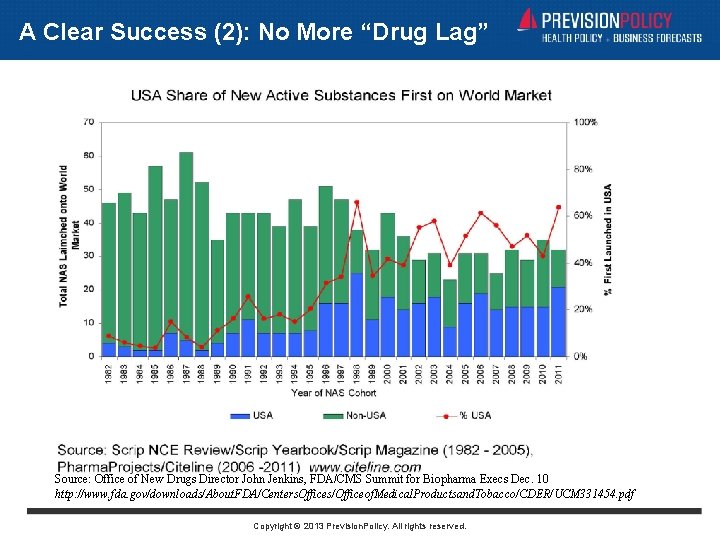
A Clear Success (2): No More “Drug Lag” Source: Office of New Drugs Director John Jenkins, FDA/CMS Summit for Biopharma Execs Dec. 10 http: //www. fda. gov/downloads/About. FDA/Centers. Offices/Officeof. Medical. Productsand. Tobacco/CDER/UCM 331454. pdf Copyright © 2013 Prevision. Policy. All rights reserved.

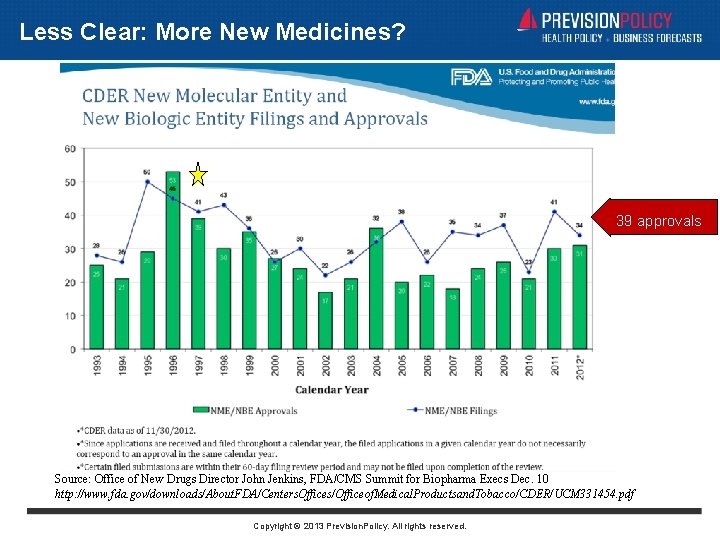
Less Clear: More New Medicines? 39 approvals Source: Office of New Drugs Director John Jenkins, FDA/CMS Summit for Biopharma Execs Dec. 10 http: //www. fda. gov/downloads/About. FDA/Centers. Offices/Officeof. Medical. Productsand. Tobacco/CDER/UCM 331454. pdf Copyright © 2013 Prevision. Policy. All rights reserved.

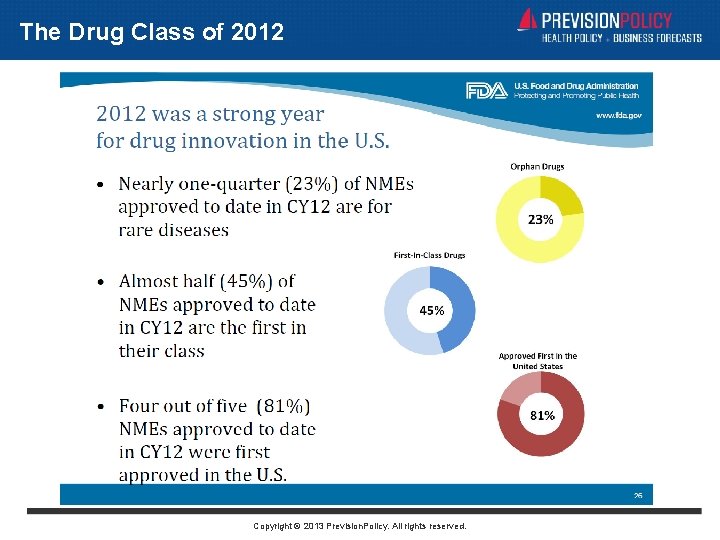
The Drug Class of 2012 Copyright © 2013 Prevision. Policy. All rights reserved.

The Year-End Approval Rush • ARIAD’s Inclusig for two rare forms of leukemia • Human Genome Sciences’ raxibacumab for inhalational anthrax • Novartis’ Signifor Cushings disease • NPS Pharma’s Gattex for short bowel syndrome • Aegerion’s Juxtapid for a rare cholesterol disorder • Bristol-Myers Squibb’s Eliquis anti-clotting drug • Janssen’s Sirturo for pulmonary tuberculosis • Salix’ Fulyzaq first drug approved for HIV-associated diarrhea Copyright © 2013 Prevision. Policy. All rights reserved.

Dueling Cartoons December 31 Going Over the Cliff January 1 We Have a Deal! Sort of…. Copyright © 2013 Prevision. Policy. All rights reserved.

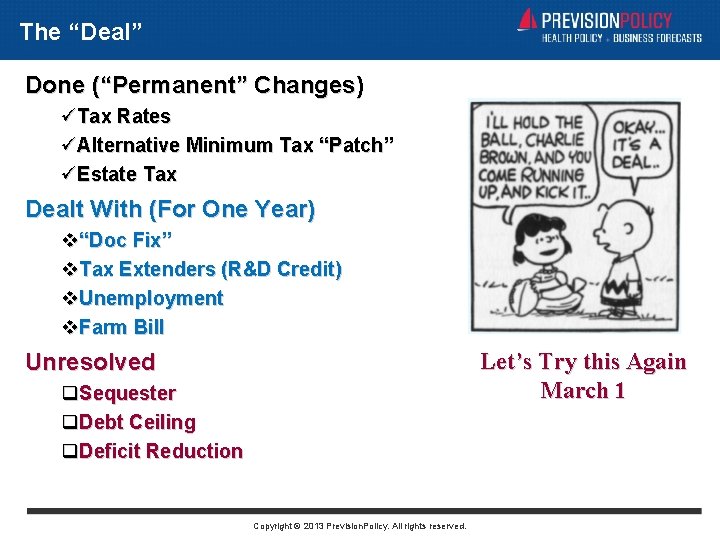
The “Deal” Done (“Permanent” Changes) üTax Rates üAlternative Minimum Tax “Patch” üEstate Tax Dealt With (For One Year) v“Doc Fix” v. Tax Extenders (R&D Credit) v. Unemployment v. Farm Bill Let’s Try this Again March 1 Unresolved q. Sequester q. Debt Ceiling q. Deficit Reduction Copyright © 2013 Prevision. Policy. All rights reserved.

A Grand Bargain in 2013? Divided Government Can Work Balanced Budget Act of 1997 Copyright © 2013 Prevision. Policy. All rights reserved.

Medicare Cuts and Pharma “Round Up The Usual Suspects” ü ü ü Part D Rebates for Duals Change in Part B Payment (ASP+4%? ) Move Part B Drugs To D Generic Incentives Least Costly Alternative End “Pay for Delay” Settlements Copyright © 2013 Prevision. Policy. All rights reserved.

Key Takeaways Health Reform Will Proceed Pharma already paid for insurance expansion in form of new fees, rebates and discounts in 2010 SCOTUS ruling on Medicaid expansion lowers number of newly covered Administration will continue to push for implementation of the Independent Payment Advisory Board; industry would need to seek separate “repeal” bill Positive FDA Environment Will Continue Seamless leadership = uninterrupted FDASIA/PDUFA V implementation Climate for new drug reviews as good as it gets; “drug lag” no more User fees increasingly critical for FDA resources FDA Congressional Oversight “Tinkering, ” Not Major Overhaul Rx compounding legislation likely for 2013 Fiscal Cliff Deal Provides 85 -Day Reprieve From Part D Rebates Biden-Mc. Connell deal excluded major provisions that would affect industry; sequestration avoided Key question will be whether Part D rebates for duals can be protected Copyright © 2013 Prevision. Policy. All rights reserved.

Thank You! Resources FDASIA Website GDUFA Website http: //www. fda. gov/For. Industry/User. Fees/Generic. Drug. User. Fees/default. htm PDUFA V Website http: //www. fda. gov/Regulatory. Information/Legislation/Federal. Food. Drugand. Cosmetic. Act. FDCAct/ Significant. Amendmentstothe. FDCAct/FDASIA/ucm 20027187. htm http: //www. fda. gov/For. Industry/User. Fees/Prescription. Drug. User. Fee/ucm 272170. htm Presentations John Jenkins, FDA/CMS Summit for Biopharma Executives Dec. 10 Gregory Geba, FDA/CMS Summit for Biopharma Executives Dec. 11 www. The. RPMReport. com www. Prevision. Policy. com Kate. Rawson@Prevision. Policy. com 202 -297 -6420 Copyright © 2013 Prevision. Policy. All rights reserved. Available on request
 Sap business one holiday request approvals
Sap business one holiday request approvals Environmental approvals branch manitoba
Environmental approvals branch manitoba Health and social care component 3
Health and social care component 3 Mental health spending
Mental health spending Health and social care unit 2
Health and social care unit 2 Accidental adulteration definition
Accidental adulteration definition Continuum of care reform
Continuum of care reform Levels of care primary secondary tertiary
Levels of care primary secondary tertiary State and local taxes and spending
State and local taxes and spending Chapter 14: taxes and government spending section 1
Chapter 14: taxes and government spending section 1 Chapter 14 taxes and government spending
Chapter 14 taxes and government spending John and marcia monthly spending plan 1 answer key
John and marcia monthly spending plan 1 answer key Why is evaluating and adjusting a spending plan important
Why is evaluating and adjusting a spending plan important Chapter 14 taxes and government spending
Chapter 14 taxes and government spending Chapter 22 lesson 1
Chapter 22 lesson 1 Otcurine
Otcurine Market forms of meat fresh meat
Market forms of meat fresh meat Scar defect in casting
Scar defect in casting Pearson
Pearson Why is carcase meat prepared into cuts joints and mince
Why is carcase meat prepared into cuts joints and mince West yorkshire health and care partnership
West yorkshire health and care partnership Humber coast and vale ics map
Humber coast and vale ics map Kerry murray
Kerry murray Verna and sam barriers
Verna and sam barriers Chapter 10 section 1 democratic reform and activism
Chapter 10 section 1 democratic reform and activism Chapter 10 section 1 democratic reform and activism
Chapter 10 section 1 democratic reform and activism Chapter 23 lesson 3 nationalism unification and reform
Chapter 23 lesson 3 nationalism unification and reform The ferment of reform and culture
The ferment of reform and culture Revolution brings reform and terror
Revolution brings reform and terror Taxation and budget reform commission
Taxation and budget reform commission Fdr three rs
Fdr three rs Chapter 19 political reform and the progressive era
Chapter 19 political reform and the progressive era Chapter 15 the ferment of reform and culture
Chapter 15 the ferment of reform and culture What was reform darwinism?
What was reform darwinism? Chapter 7 section 2 revolution brings reform and terror
Chapter 7 section 2 revolution brings reform and terror Chapter 15 the ferment of reform and culture
Chapter 15 the ferment of reform and culture Chapter 12: religion, romanticism, and reform, 1800–1860
Chapter 12: religion, romanticism, and reform, 1800–1860 Chapter 23 section 2 revolution brings reform and terror
Chapter 23 section 2 revolution brings reform and terror