Health Care Compliance Assoc 2000 Compliance Institute Valli


















- Slides: 19

Health Care Compliance Assoc. 2000 Compliance Institute Valli Baldassano Pharmacia Corporation

On the Agenda • • History Dose of Reality The Arsenal Take Aways

History • 1991 OIG Study: “Promotion of Prescription Drugs Through Payments and Gifts” – Approved product studies are schemes to pay doctors • 1993 & 1994 State enforcement against “Switching Programs” – Miles pays $805, 000 and Upjohn pays $675, 000 • cognitive services programs to pharmacists

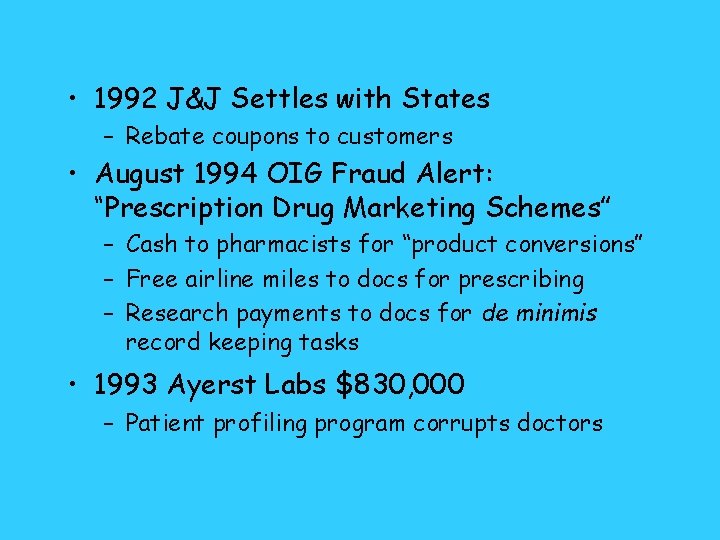
• 1992 J&J Settles with States – Rebate coupons to customers • August 1994 OIG Fraud Alert: “Prescription Drug Marketing Schemes” – Cash to pharmacists for “product conversions” – Free airline miles to docs for prescribing – Research payments to docs for de minimis record keeping tasks • 1993 Ayerst Labs $830, 000 – Patient profiling program corrupts doctors

• 1994 Rugby $200, 000 – Pharmacists get vacation packages for buying product • 1994 Hoffman La Roche $450, 000 – Small grants to doctors for sham research on FDA approved products • 1995 Merck-Medco – Consent decree: full disclosure of relationship with Merck and switching programs

• 1995 Caremark International $161, 000 – Kickback claims that took several forms: research grants, consulting agreements and paying for office expenses

Recent Activity • March 1999 Novartis (Ciba-Geigy) $8, 000 – Overcharged VA by failing to provide accurate pricing information during negotiations • May, 1999 Genetech $50, 000 – Off-label promotion of Protropin, allegedly facilitated through educational grants and sham research payments • May, 1999 NMC $16, 500, 000 – Kickbacks for diagnotic tests – NMC president & vice president indicted

• January, 2000 Fresenius Medical Care $486, 000 (including $101, 000 criminal fines) – False claims/ giving educational grants and other payments as kickbacks for business • California allergist indicted (winter 2000) – Study for RPR faked • Bindley Western 4/2000 $25, 800, 000 – (criminal) rogue employees of wholesaler diverting drugs through gray market

• Bayer and others (reported 5/2000) – Deceptive Pricing Practices • AWP • Medicaid Best Price – repackaging – Federal and State Enforcement – Congressional Inquiry

• Warner Lambert (reported 5/2000) – Whistleblower suit involving promotion of WL’s epilepsy drug • PBM Investigation (reported March 2000) – Switching Programs – Upstream and Downstream Rebates – Formulary Access

Government Aresenal • Anti-kickback Statute 42 U. S. C. § 1320 a 7 b(b) – Illegal remuneration to influence purchase, prescription or recommendation of any item reimburseable by Medicare or Medicaid – “One Purpose” Test • liability if only one purpose of payment is to influence. U. S. v. Greber, 760 F. 2 d 68, 69 (3 d Cir. 1985)

Kickbacks can be • • • . . . wining and dining consulting services advisory boards clinical studies free goods business assistance charitable/public service expenditures discounts and rebates bundling arrangements

No Easy Escape Through Safe Harbors • • • Discount Bona fide Employees Group Purchasing Organizations Personal Services Shared Risk

False Claims Act • Civil liability for person who knowingly presents or causes to be presented a false or fraudulent claim for payment. 31 U. S. C. § 3729 – Home of the whistleblower – Industry practice under attack: AWP and Medicaid Best Price

How Can AWP Reporting be a False Claim? ! • Claim is Facially False – AWP not defined by regulation and widely published – Would have to prove knowledge of or willful blindness to doctor’s failure to report discounts • questionable whether doctors are required to report discounts

• “Fraudulent Implied Certification” Theory – Claimant implicitly certifies compliance with the law when submitting claim. • U. S. ex rel. Pogue v. Healthcorp, Inc. , 914 F. Supp. 1507 (M. D. Tenn. 1996) – Liability attaches only if compliance with regulations at issue is a prerequisite for obtaining the benefit and the defendant affirmatively certified such compliance • Harrison v. Westinghouse Savannah River Co. , 176 F. 3 d 776, 778 (4 th Cir. 1999) • Thompson v. Columbia/HCA Healthcare Corp. , 125 F. 3 d 899, 902 (5 th Cir. 1997)

• “Fraudulent Course of Conduct” Theory – Fraudulent course of conduct caused government to pay claim for money • U. S. v. Incorp Village of Island Park, 888 F. Supp. 419, 439 (E. D. N. Y. 1995) – FCA “reaches beyond ‘claims’ which might be legally enforced, to all fraudulent attempts to cause the government to pay out sums of money. ” • U. S. v. Mc. Leod, 721 F. 2 d 282, 284 (9 th Cir. 1983) • U. S. Neifert-White Co. , 390 U. S. 228, 233 (1968)

A Note on PBMs. . . • FCA potentially applies to allegations: – – – skimming discounts to pharmacies “playing the float” billing for services not provided brand/generic switch & bill switch programs • Kickbacks in procurement: 41 U. S. C. § 51 or Robinson Patman § 2(c) – formulary inclusion

Take Aways • Pharma Initiatives Are Here – Medicare Drug Benefit – Congressional hearings – Public proclamations • The Rules are Being Redefined • Difficult to Advise Clients • Compliance Programs Make Sense
 Common lisp assoc
Common lisp assoc Aaa gestation calculator
Aaa gestation calculator Sakal kushal valli
Sakal kushal valli Dora dymant
Dora dymant Valli sarveswaran
Valli sarveswaran Ilkka valli
Ilkka valli Laura valli anac
Laura valli anac Australian primary health care research institute
Australian primary health care research institute Interagency institute for federal health care executives
Interagency institute for federal health care executives Primary secondary tertiary health care definition
Primary secondary tertiary health care definition Health and social care values unit 2
Health and social care values unit 2 Health and social care component 3 health and wellbeing
Health and social care component 3 health and wellbeing Office of health standards compliance
Office of health standards compliance Compliance motivation and health behaviors of the learner
Compliance motivation and health behaviors of the learner Telethon institute for child health research
Telethon institute for child health research Symbiosis hospital management
Symbiosis hospital management Public health institute nagpur
Public health institute nagpur Roadmap to success
Roadmap to success Reach institute mental health
Reach institute mental health California institute for mental health
California institute for mental health





































