Groups of Minerals Liz La Rosa 5 th










- Slides: 19

Groups of Minerals Liz La. Rosa 5 th grade science www. middleschoolscience. com 2010

Mineral Groups - Notes As you view the slides, write your notes into the graphic organizers. Include all the information from each slide into the appropriate boxes. Write neatly and carefully. You will not be expected to memorize this information, but this information will be used as a reference throughout our mineral unit and help you become familiar with the different types of minerals that exist.

Mineral Groups Minerals are divided into groups based on their chemical composition The two main groups all minerals can be classified into are: ▪ Silicates ▪ Nonsilicates

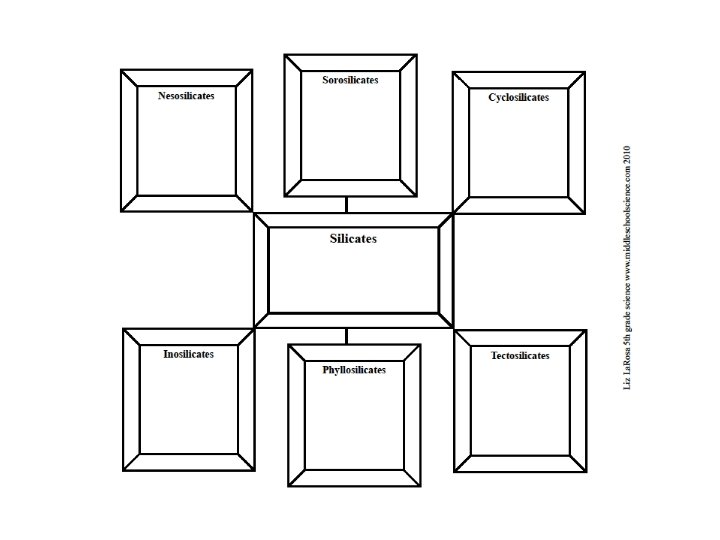
Silicates Made of Silicon (Si) and Oxygen (O) ▪ Example: Si. O 4 Make up 90% of the Earth’s crust Combine with elements such as Aluminum (Al), Iron (Fe), Magnesium (Mg), and Potassium (K)

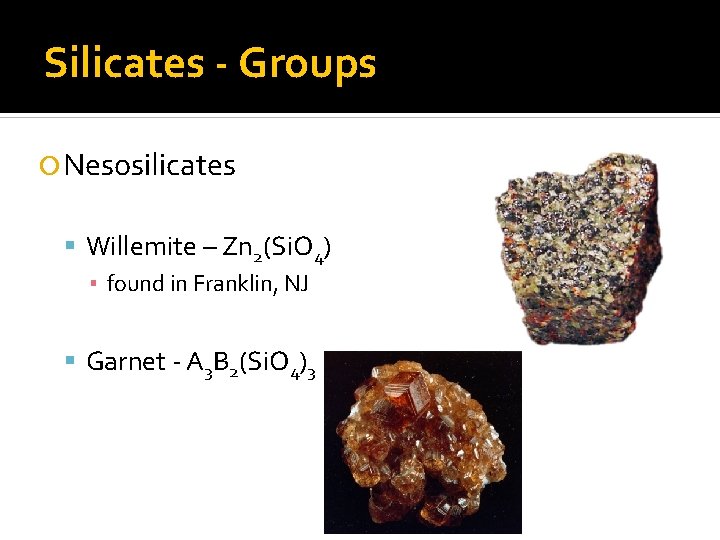
Silicates - Groups Nesosilicates Willemite – Zn 2(Si. O 4) ▪ found in Franklin, NJ Garnet - A 3 B 2(Si. O 4)3

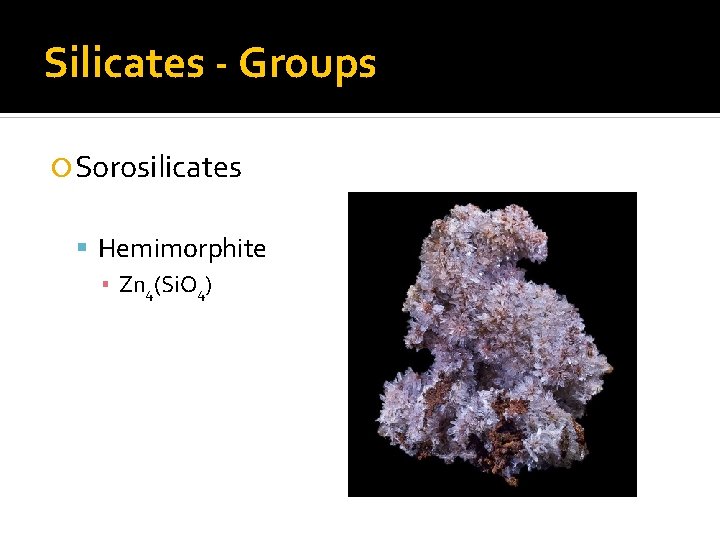
Silicates - Groups Sorosilicates Hemimorphite ▪ Zn 4(Si. O 4)

Silicates - Groups Cyclosilicates Beryl ▪ Be 3 Al 2(Si 6 O 18)

Silicates - Groups Inosilicates Hornblende ▪ (Ca, Na)2(Mg, Fe, Al)5(Al, Si)8 O 22 (OH)2

Silicates - Groups Phyllosilicates Talc ▪ Mg 3(Si 4 O 10)(OH)2

Silicates - Groups Tectosilicates Quartz ▪ Si. O 2 Opal ▪ Si. O 2. n. H 2 O

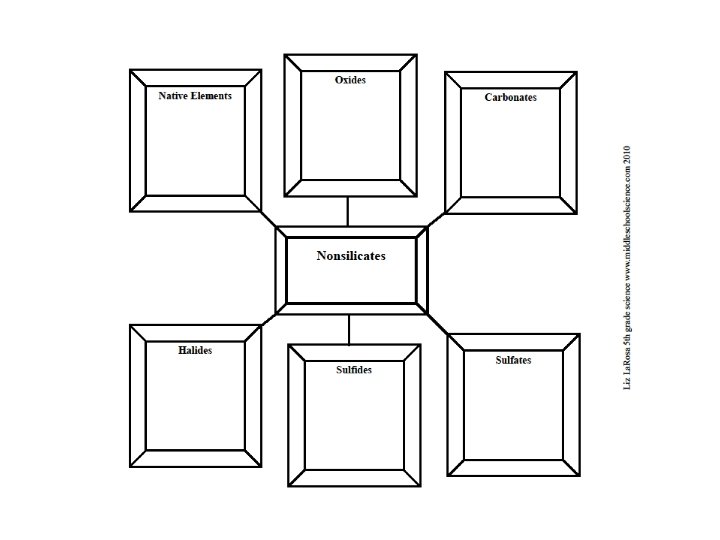
Nonsilicates Do not contain a combination of Silicon (Si) and Oxygen (O) in their chemical composition There are 6 groups of nonsilicates: 1. 2. 3. 4. 5. 6. Native Elements Oxides Carbonates Sulfates Halides Sulfides

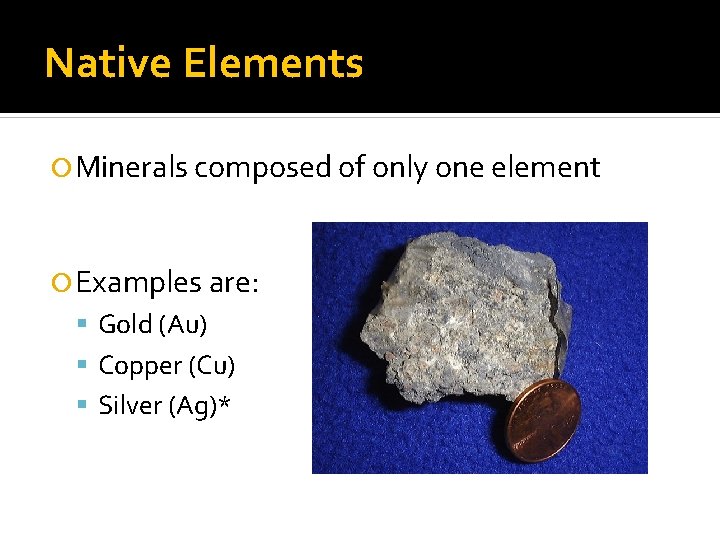
Native Elements Minerals composed of only one element Examples are: Gold (Au) Copper (Cu) Silver (Ag)*

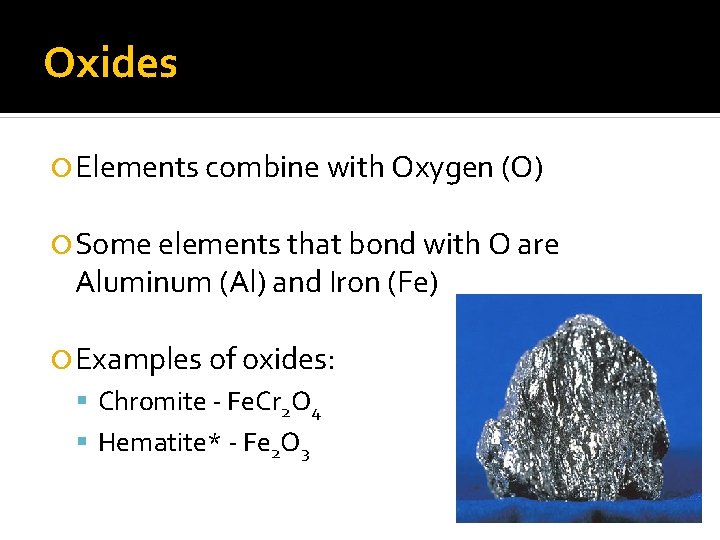
Oxides Elements combine with Oxygen (O) Some elements that bond with O are Aluminum (Al) and Iron (Fe) Examples of oxides: Chromite - Fe. Cr 2 O 4 Hematite* - Fe 2 O 3

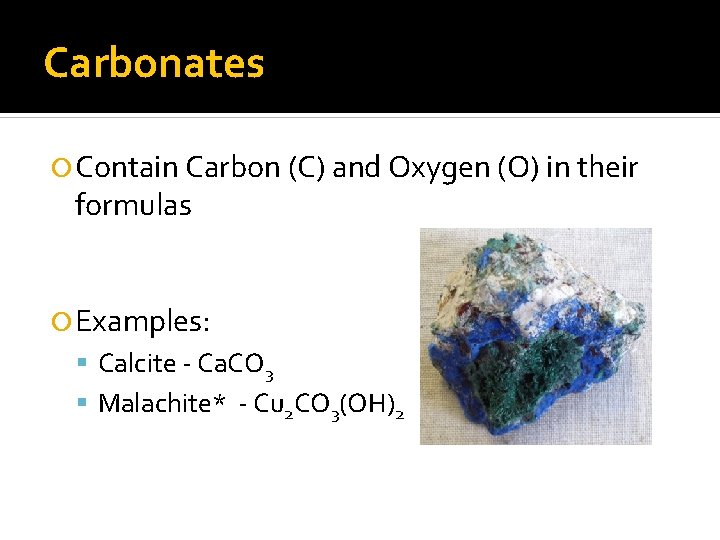
Carbonates Contain Carbon (C) and Oxygen (O) in their formulas Examples: Calcite - Ca. CO 3 Malachite* - Cu 2 CO 3(OH)2

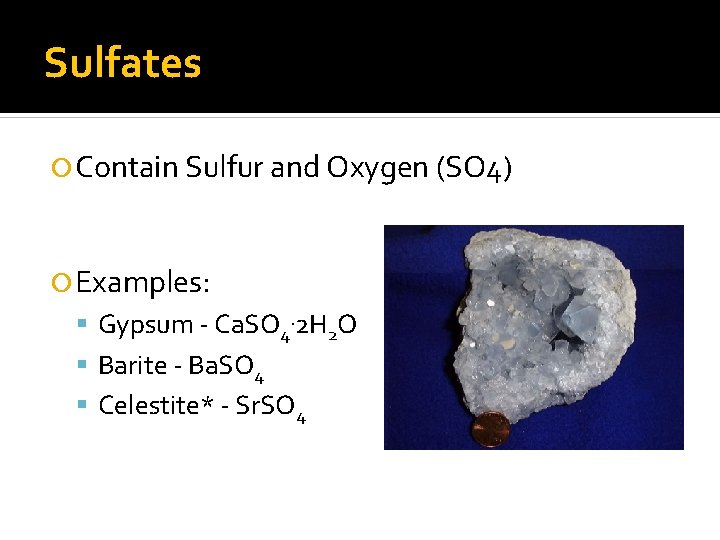
Sulfates Contain Sulfur and Oxygen (SO 4) Examples: Gypsum - Ca. SO 4. 2 H 2 O Barite - Ba. SO 4 Celestite* - Sr. SO 4

Halides Compounds formed when Fluorine (F), Chlorine (Cl), Iodine (I), or Bromine (Br) bond with Sodium (Na), Potassium (K), or Calcium (Ca) Examples: Halite - Na. Cl Fluorite - Ca. F 2

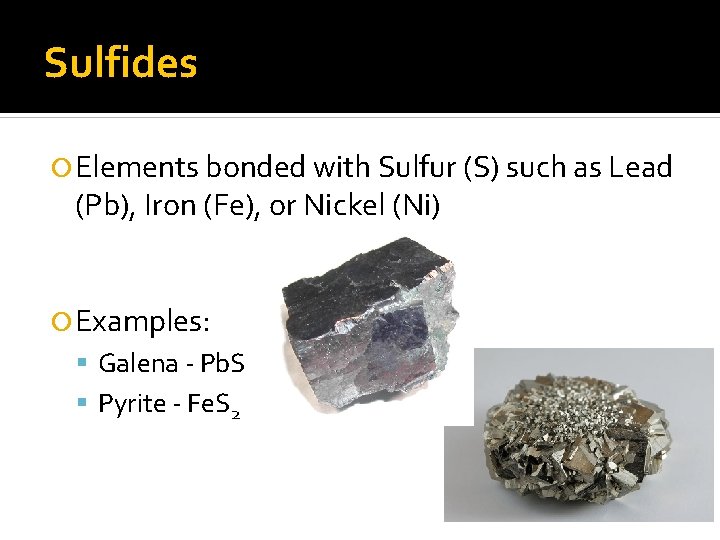
Sulfides Elements bonded with Sulfur (S) such as Lead (Pb), Iron (Fe), or Nickel (Ni) Examples: Galena - Pb. S Pyrite - Fe. S 2


 Rosangela rosa da rosa
Rosangela rosa da rosa How are ethnic groups and religious groups related
How are ethnic groups and religious groups related Liz smith nurse
Liz smith nurse Symbols in the poisonwood bible
Symbols in the poisonwood bible Liz sneddon probability
Liz sneddon probability Liz sneddon bivariate
Liz sneddon bivariate Liz martindale
Liz martindale Riot games slogan
Riot games slogan Liz found organizing her closet such a
Liz found organizing her closet such a Liz nichols art
Liz nichols art Good morning elizabeth
Good morning elizabeth Liz duff
Liz duff Liz seabury principal
Liz seabury principal Www middleschoolscience com 2008
Www middleschoolscience com 2008 Liz alessi
Liz alessi Revelation liz lochhead analysis
Revelation liz lochhead analysis The thickness of ice poem analysis
The thickness of ice poem analysis The bargain liz lochhead
The bargain liz lochhead Liz keogh
Liz keogh Liz pellicano
Liz pellicano