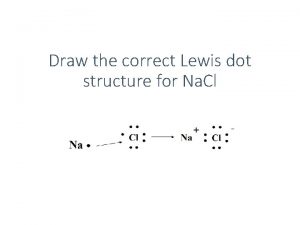
Gram to Gram Conversions Aluminum is an active










- Slides: 25

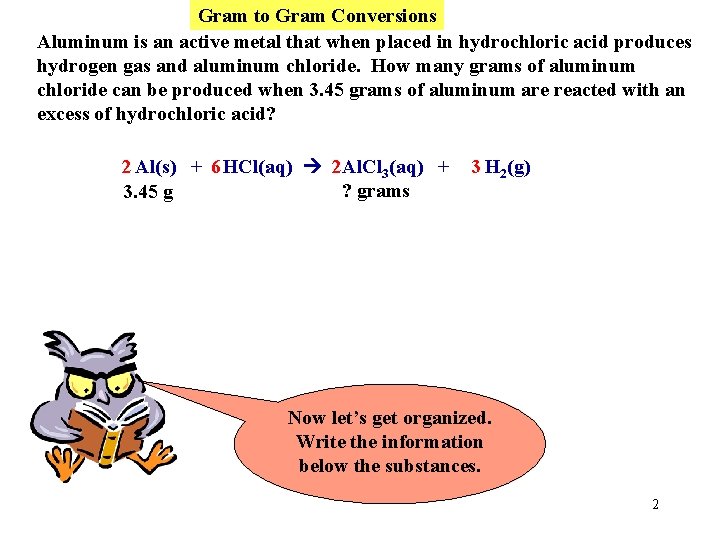
Gram to Gram Conversions Aluminum is an active metal that when placed in hydrochloric acid produces hydrogen gas and aluminum chloride. How many grams of aluminum chloride can be produced when 3. 45 grams of aluminum are reacted with an excess of hydrochloric acid? 2 Al(s) + 6 HCl(aq) 2 Al. Cl 3(aq) + 3 H 2(g) First write a balanced equation. 1

Gram to Gram Conversions Aluminum is an active metal that when placed in hydrochloric acid produces hydrogen gas and aluminum chloride. How many grams of aluminum chloride can be produced when 3. 45 grams of aluminum are reacted with an excess of hydrochloric acid? 2 Al(s) + 6 HCl(aq) 2 Al. Cl 3(aq) + ? grams 3. 45 g 3 H 2(g) Now let’s get organized. Write the information below the substances. 2

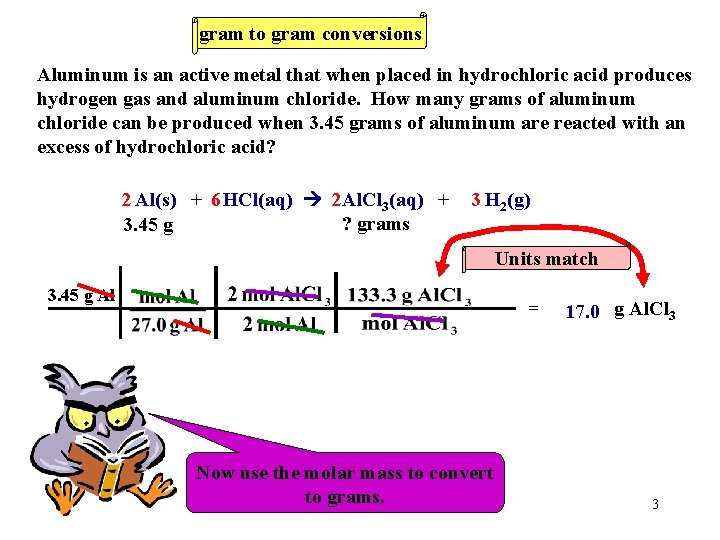
gram to gram conversions Aluminum is an active metal that when placed in hydrochloric acid produces hydrogen gas and aluminum chloride. How many grams of aluminum chloride can be produced when 3. 45 grams of aluminum are reacted with an excess of hydrochloric acid? 2 Al(s) + 6 HCl(aq) 2 Al. Cl 3(aq) + ? grams 3. 45 g 3 H 2(g) Units match 3. 45 g Al = Now We must Now use Let’s the always use work molar thethe convert molar mass problem. ratio. to toconvert moles. to grams. 17. 0 g Al. Cl 3 3

4

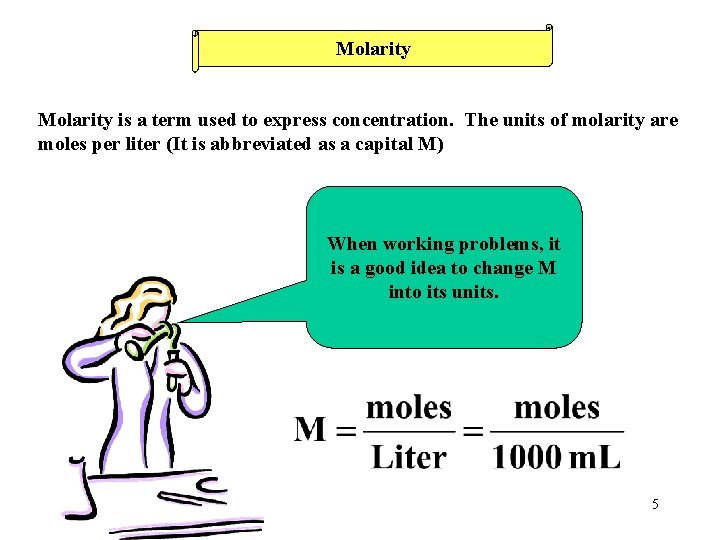
Molarity is a term used to express concentration. The units of molarity are moles per liter (It is abbreviated as a capital M) When working problems, it is a good idea to change M into its units. 5

6

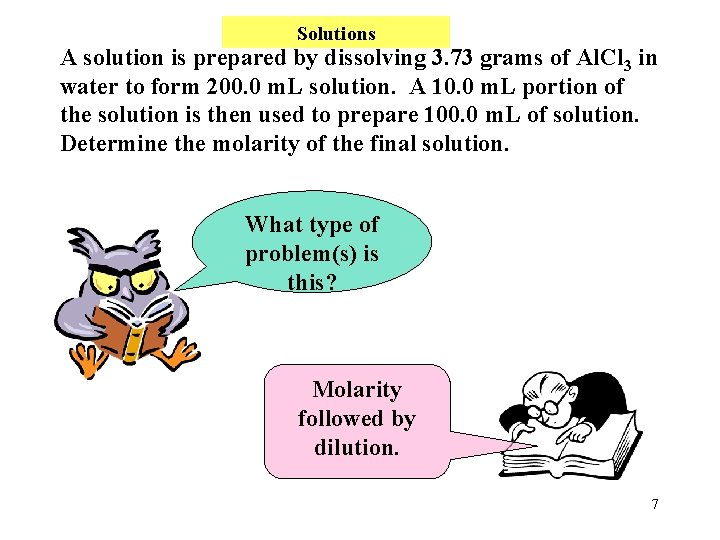
Solutions A solution is prepared by dissolving 3. 73 grams of Al. Cl 3 in water to form 200. 0 m. L solution. A 10. 0 m. L portion of the solution is then used to prepare 100. 0 m. L of solution. Determine the molarity of the final solution. What type of problem(s) is this? Molarity followed by dilution. 7

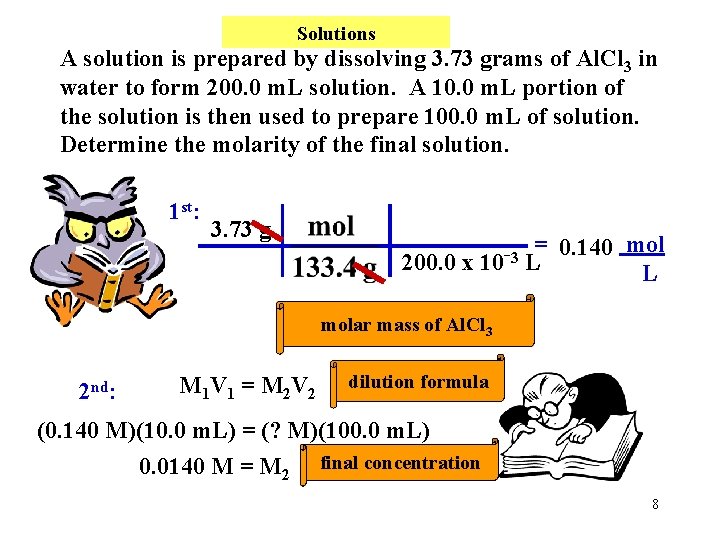
Solutions A solution is prepared by dissolving 3. 73 grams of Al. Cl 3 in water to form 200. 0 m. L solution. A 10. 0 m. L portion of the solution is then used to prepare 100. 0 m. L of solution. Determine the molarity of the final solution. 1 st: 3. 73 g = 0. 140 mol 3 200. 0 x 10 L L molar mass of Al. Cl 3 2 nd: M 1 V 1 = M 2 V 2 dilution formula (0. 140 M)(10. 0 m. L) = (? M)(100. 0 m. L) 0. 0140 M = M 2 final concentration 8

9

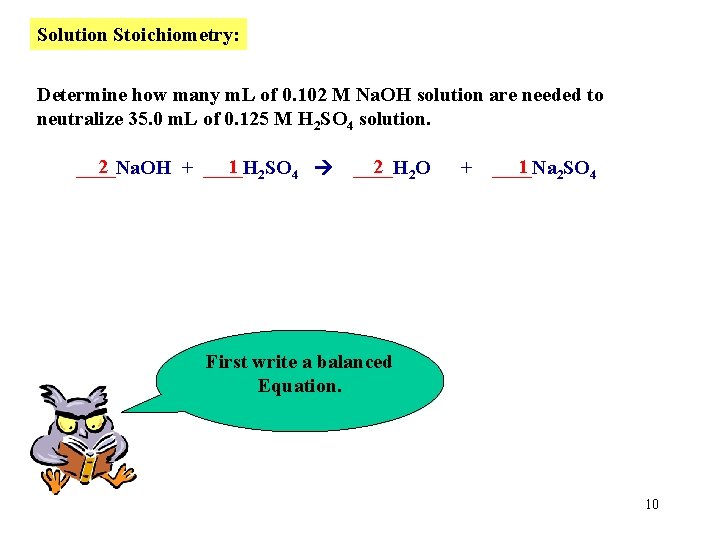
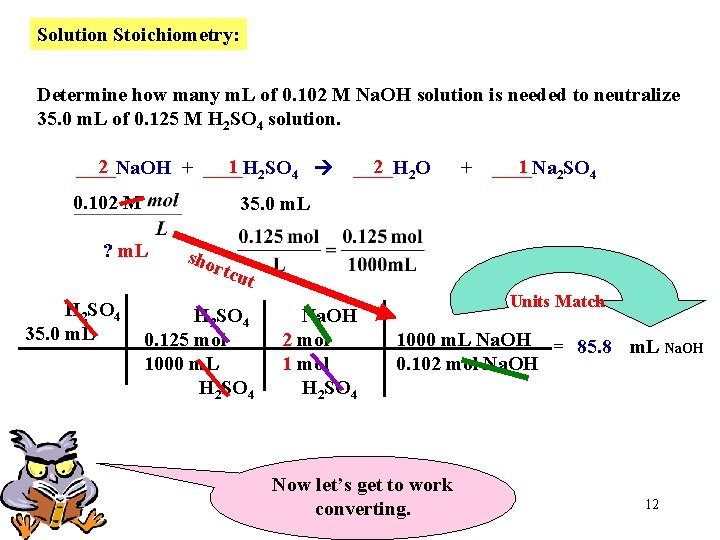
Solution Stoichiometry: Determine how many m. L of 0. 102 M Na. OH solution are needed to neutralize 35. 0 m. L of 0. 125 M H 2 SO 4 solution. 2 1 2 SO 4 ____Na. OH + ____H 2 2 O ____H + 1 2 SO 4 ____Na First write a balanced Equation. 10

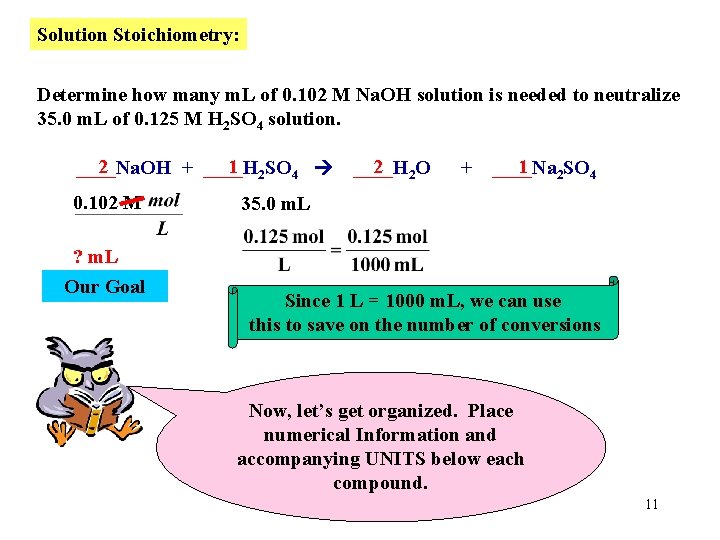
Solution Stoichiometry: Determine how many m. L of 0. 102 M Na. OH solution is needed to neutralize 35. 0 m. L of 0. 125 M H 2 SO 4 solution. 2 1 2 SO 4 ____Na. OH + ____H 0. 102 M 2 2 O ____H + 1 2 SO 4 ____Na 35. 0 m. L ? m. L Our Goal Since 1 L = 1000 m. L, we can use this to save on the number of conversions Now, let’s get organized. Place numerical Information and accompanying UNITS below each compound. 11

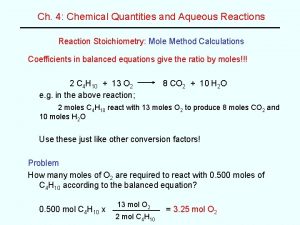
Solution Stoichiometry: Determine how many m. L of 0. 102 M Na. OH solution is needed to neutralize 35. 0 m. L of 0. 125 M H 2 SO 4 solution. 2 1 2 SO 4 ____Na. OH + ____H 0. 102 M + 1 2 SO 4 ____Na 35. 0 m. L ? m. L H 2 SO 4 35. 0 m. L 2 2 O ____H sho rtcu t H 2 SO 4 0. 125 mol 1000 m. L H 2 SO 4 Na. OH 2 mol 1 mol H 2 SO 4 Units Match 1000 m. L Na. OH = 85. 8 m. L Na. OH 0. 102 mol Na. OH Now let’s get to work converting. 12

13

Solution Stoichiometry What volume of 0. 40 M HCl solution is needed to completely neutralize 47. 1 m. L of 0. 75 M Ba(OH)2? 1 st write out a balanced chemical equation 14

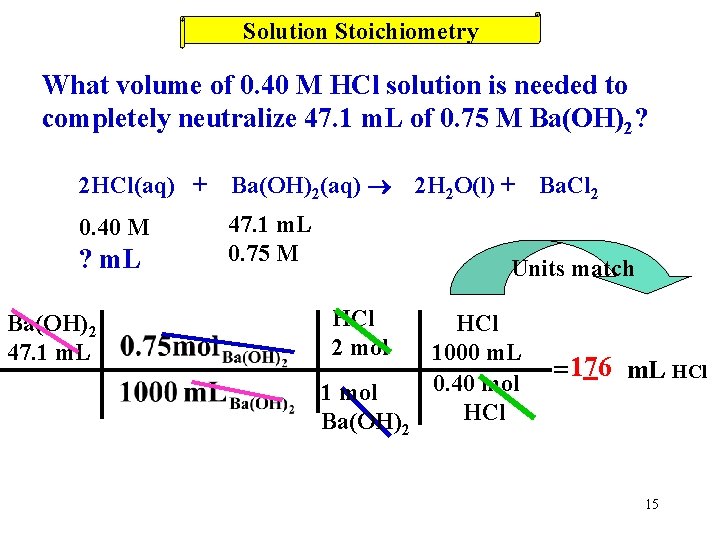
Solution Stoichiometry What volume of 0. 40 M HCl solution is needed to completely neutralize 47. 1 m. L of 0. 75 M Ba(OH)2? 2 HCl(aq) + Ba(OH)2(aq) 0. 40 M 47. 1 m. L 0. 75 M ? m. L Ba(OH)2 47. 1 m. L 2 H 2 O(l) + Ba. Cl 2 Units match HCl 2 mol 1 mol Ba(OH)2 HCl 1000 m. L 0. 40 mol HCl = 176 m. L HCl 15

16

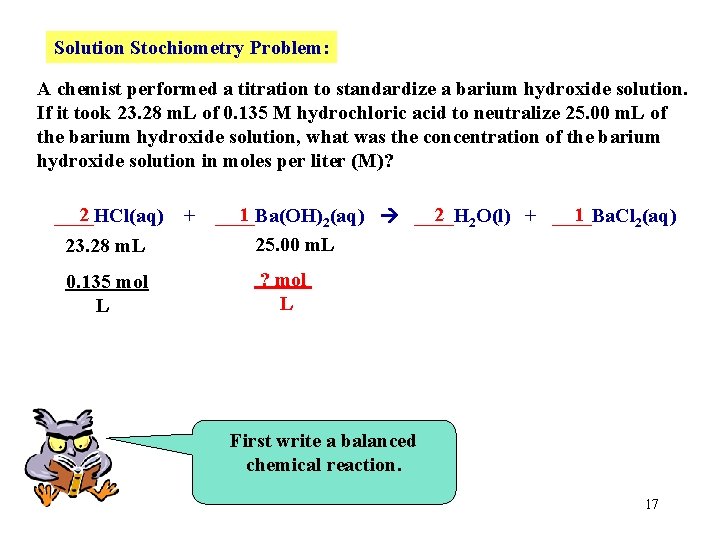
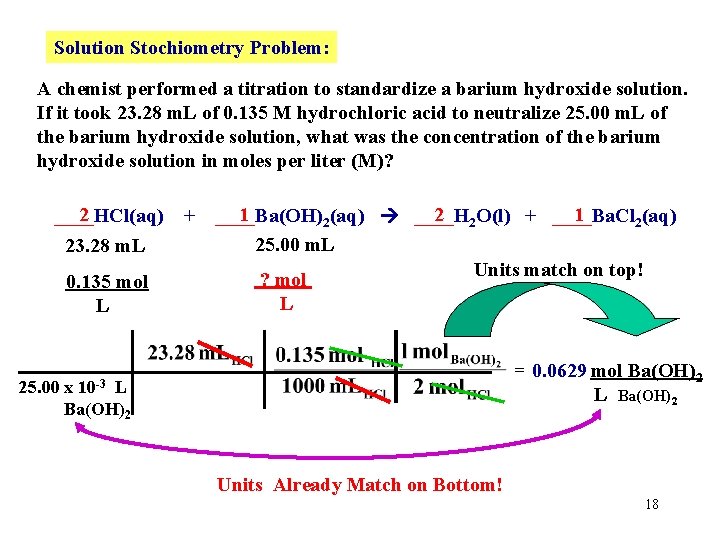
Solution Stochiometry Problem: A chemist performed a titration to standardize a barium hydroxide solution. If it took 23. 28 m. L of 0. 135 M hydrochloric acid to neutralize 25. 00 m. L of the barium hydroxide solution, what was the concentration of the barium hydroxide solution in moles per liter (M)? 2 ____HCl(aq) 23. 28 m. L 0. 135 mol L + 1 2 2 O(l) + ____Ba. Cl 1 ____Ba(OH) 2(aq) ____H 2(aq) 25. 00 m. L ? mol L First write a balanced chemical reaction. 17

Solution Stochiometry Problem: A chemist performed a titration to standardize a barium hydroxide solution. If it took 23. 28 m. L of 0. 135 M hydrochloric acid to neutralize 25. 00 m. L of the barium hydroxide solution, what was the concentration of the barium hydroxide solution in moles per liter (M)? 2 ____HCl(aq) 23. 28 m. L 0. 135 mol L + 1 2 2 O(l) + ____Ba. Cl 1 ____Ba(OH) 2(aq) ____H 2(aq) 25. 00 m. L Units match on top! ? mol L = 0. 0629 mol Ba(OH)2 L Ba(OH)2 10 -3 25. 00 x L Ba(OH)2 Units Already Match on Bottom! 18

19

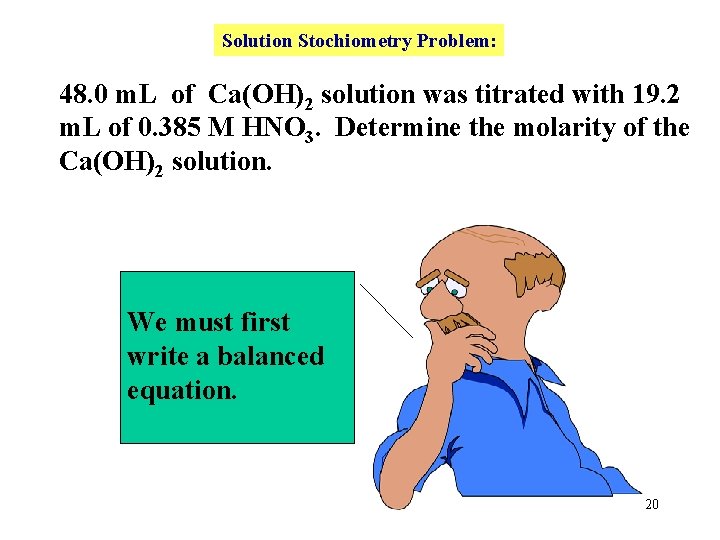
Solution Stochiometry Problem: 48. 0 m. L of Ca(OH)2 solution was titrated with 19. 2 m. L of 0. 385 M HNO 3. Determine the molarity of the Ca(OH)2 solution. We must first write a balanced equation. 20

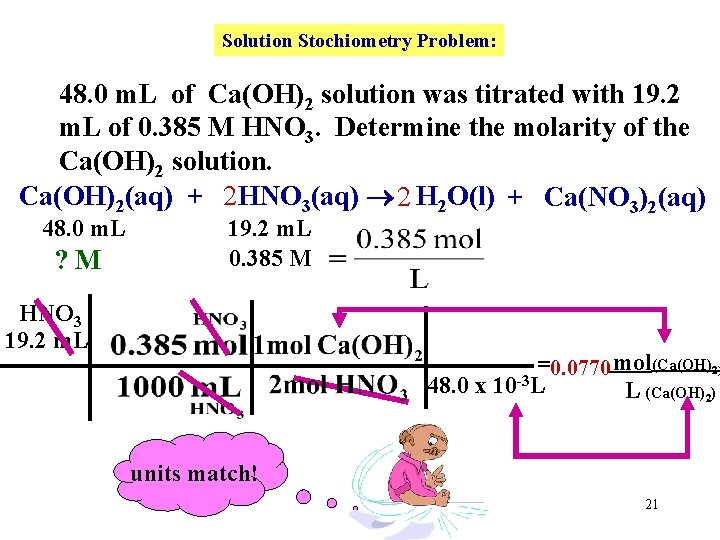
Solution Stochiometry Problem: 48. 0 m. L of Ca(OH)2 solution was titrated with 19. 2 m. L of 0. 385 M HNO 3. Determine the molarity of the Ca(OH)2 solution. Ca(OH)2(aq) + 2 HNO 3(aq) 2 H 2 O(l) + Ca(NO 3)2(aq) 48. 0 m. L ? M 19. 2 m. L 0. 385 M HNO 3 19. 2 m. L =0. 0770 mol(Ca(OH)2) 48. 0 x 10 -3 L L (Ca(OH)2) units match! 21

22

Limiting/Excess/ Reactant and Theoretical Yield Problems : Potassium superoxide, KO 2, is used in rebreathing gas masks to generate oxygen. 4 KO 2(s) + 2 H 2 O(l) 4 KOH(s) + 3 O 2(g) a. How many moles of O 2 can be produced from 0. 15 mol KO 2 and 0. 10 mol H 2 O? b. Determine the limiting reactant. 4 KO 2(s) + 2 H 2 O(l) 4 KOH(s) + 3 O 2(g) 0. 15 mol ? moles 0. 10 mol Hide Two starting amounts? Where do we start? one 23

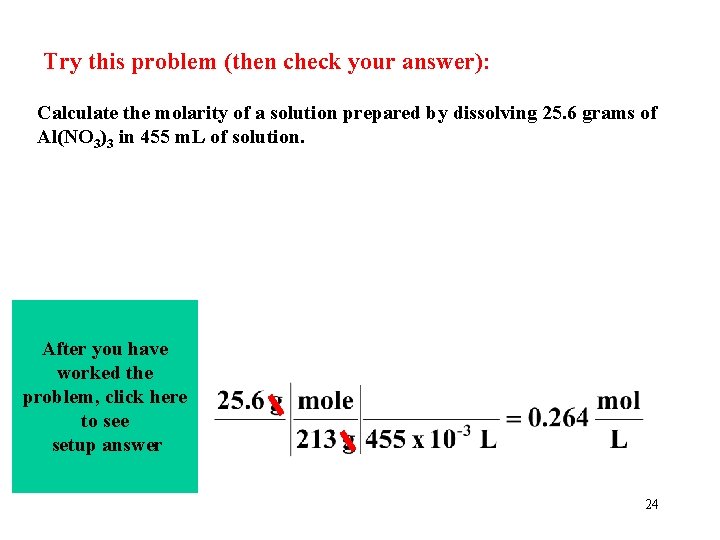
Try this problem (then check your answer): Calculate the molarity of a solution prepared by dissolving 25. 6 grams of Al(NO 3)3 in 455 m. L of solution. After you have worked the problem, click here to see setup answer 24

25
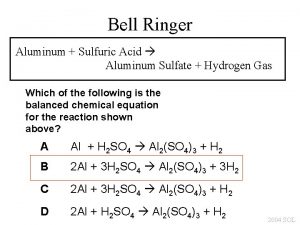
 Aluminum + sulfuric acid = aluminum sulfate + hydrogen
Aluminum + sulfuric acid = aluminum sulfate + hydrogen Mole-mole stoichiometry
Mole-mole stoichiometry Buktikan rumus rumus berikut benar secara dimensi
Buktikan rumus rumus berikut benar secara dimensi 20 gram fenol dicampur dengan 30 gram air
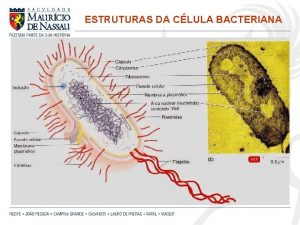
20 gram fenol dicampur dengan 30 gram air Endoflagelos
Endoflagelos Cuadro comparativo entre gram positivas y gram negativas
Cuadro comparativo entre gram positivas y gram negativas Eubatteri
Eubatteri 30 gram asam asetat (bm=60) dilarutkan dalam 45 gram air
30 gram asam asetat (bm=60) dilarutkan dalam 45 gram air Mesofase pada padatan dapat berupa
Mesofase pada padatan dapat berupa Bakteri gram positif dan negatif
Bakteri gram positif dan negatif Bactrias
Bactrias Membrane structures that function in active transport
Membrane structures that function in active transport Primary active transport vs secondary active transport
Primary active transport vs secondary active transport Boolean operators
Boolean operators An aluminum shaft with a constant diameter of 50mm
An aluminum shaft with a constant diameter of 50mm Bohr model vs electron cloud model
Bohr model vs electron cloud model Aluminum sulfate and calcium hydroxide
Aluminum sulfate and calcium hydroxide Electron beam welding aluminium
Electron beam welding aluminium Is cutting aluminum foil a chemical change
Is cutting aluminum foil a chemical change Easter island chile
Easter island chile Lewis dot structure for potassium
Lewis dot structure for potassium How to build a aluminum foil boat
How to build a aluminum foil boat Grams to moles examples
Grams to moles examples Skc aluminum cyclone
Skc aluminum cyclone A neutral atom of aluminum 27 contains
A neutral atom of aluminum 27 contains Aluminum and oxygen ionic compound formula
Aluminum and oxygen ionic compound formula