Atomic Structure and Periodic Relationships 2 3 ns









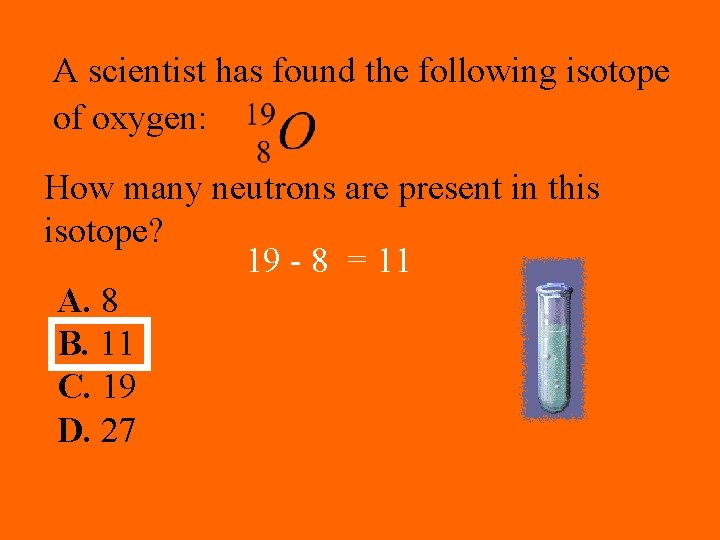



- Slides: 44

Atomic Structure and Periodic Relationships

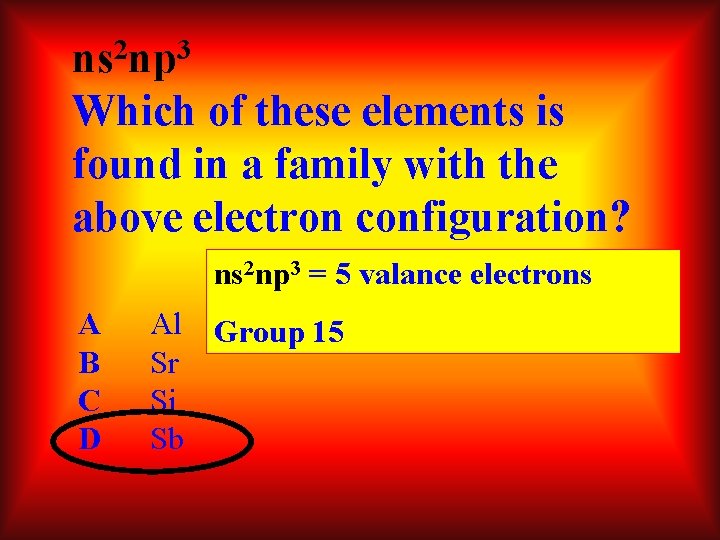
2 3 ns np Which of these elements is found in a family with the above electron configuration? ns 2 np 3 = 5 valance electrons A B C D Al Group 15 Sr Si Sb

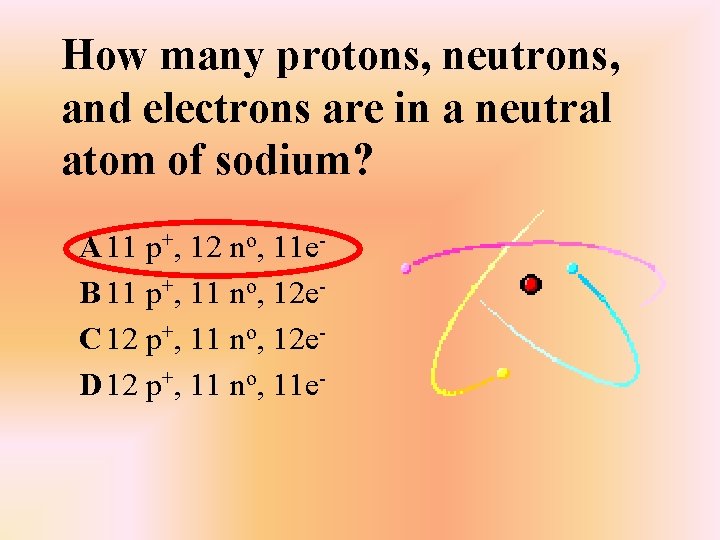
How many protons, neutrons, and electrons are in a neutral atom of sodium? A 11 p+, 12 no, 11 e. B 11 p+, 11 no, 12 e. C 12 p+, 11 no, 12 e. D 12 p+, 11 no, 11 e-

Which of the following describes what takes place when 0 2+ iron (Fe ) is oxidized to Fe ions? A B C D A gain of two electrons A loss of two electrons A gain of two protons A loss of two protons + ions lose electrons - Ions gain electrons

Which scientist was the first to conclude through experimentation that atoms have positive charges in their nuclei? A. Bohr B. Dalton C. Mosley D. Rutherford

Which grouping identifies chemical properties? A. Malleability, ductility, conductivity Physical B. Luster, hardness, texture Physical C. Combustibility, flammability, reactivity D. Density, melting point, boiling point Physical

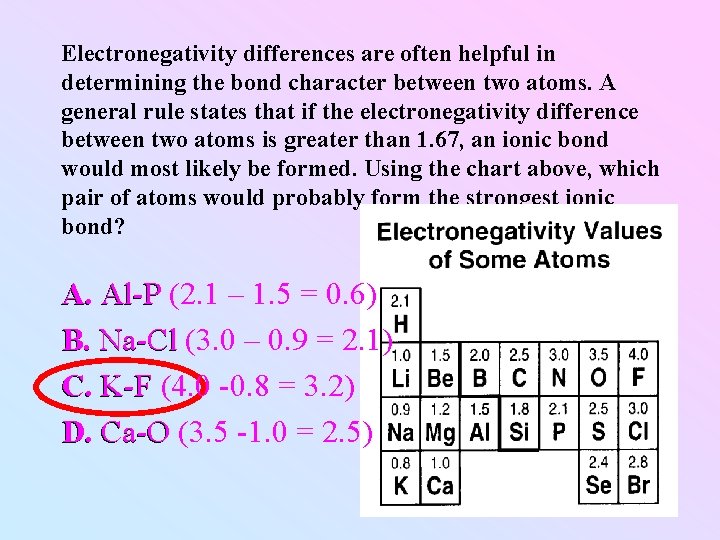
Electronegativity differences are often helpful in determining the bond character between two atoms. A general rule states that if the electronegativity difference between two atoms is greater than 1. 67, an ionic bond would most likely be formed. Using the chart above, which pair of atoms would probably form the strongest ionic bond? A. Al-P (2. 1 – 1. 5 = 0. 6) A. Al-P B. Na-Cl (3. 0 – 0. 9 = 2. 1) B. Na-Cl C. K-F (4. 0 -0. 8 = 3. 2) C. K-F D. Ca-O (3. 5 -1. 0 = 2. 5) D. Ca-O

Three elements, X, Y, and Z, have consecutive increasing atomic numbers. If element X is a noble gas, what will be the symbol for the ion of element Z in its compounds? A. Z 2 B. ZC. Z+ D. Z 2+ Group 2 = 2+

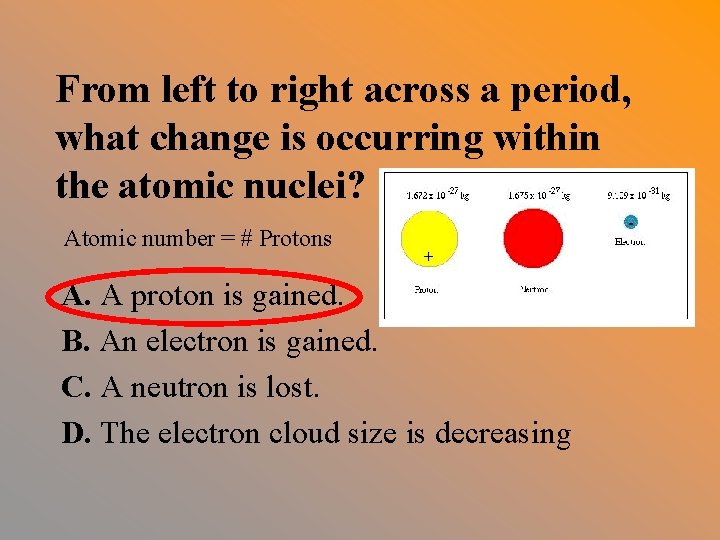
From left to right across a period, what change is occurring within the atomic nuclei? Atomic number = # Protons A. A proton is gained. B. An electron is gained. C. A neutron is lost. D. The electron cloud size is decreasing

Radioactive iodine-131 has a halflife of eight days. The amount of a 200. 0 gram sample left after 32 days would be — A. 6. 25 g B. 12. 5 g C. 25. 0 g D. 50. 0 g

Radioactive iodine-131 has a halflife of eight days. The amount of a 200. 0 gram sample left after 32 days would be —

Radioactive iodine-131 has a halflife of eight days. The amount of a 200. 0 gram sample left after 32 days would be — A. 6. 25 g B. 12. 5 g C. 25. 0 g D. 50. 0 g

Which of these describes a tendency for atomic radii as displayed on the periodic chart? A. Atomic radii decrease left to right across a period. B. Atomic radii increase left to right across a period. C. Atomic radii decrease top to bottom down a group. D. Atomic radii increase, then decrease from top to bottom down a group.

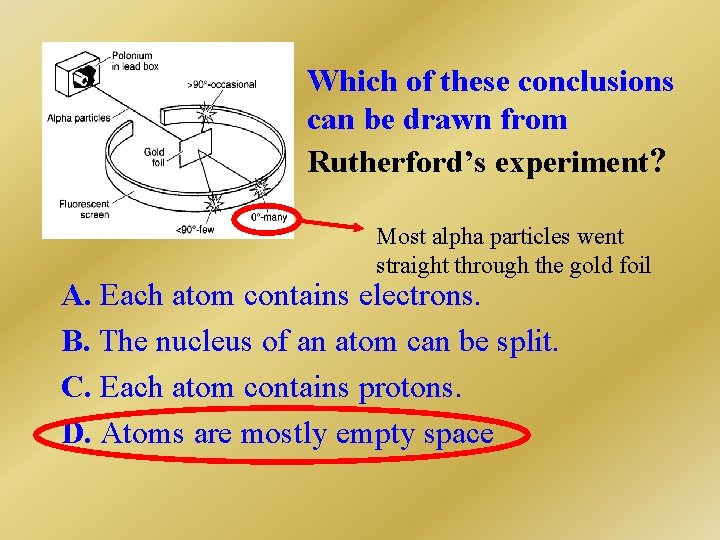
Which of these conclusions can be drawn from Rutherford’s experiment? Most alpha particles went straight through the gold foil A. Each atom contains electrons. B. The nucleus of an atom can be split. C. Each atom contains protons. D. Atoms are mostly empty space

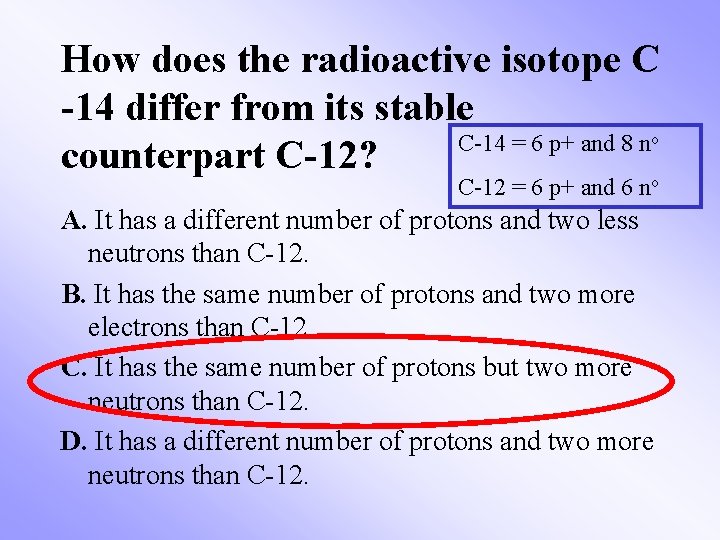
How does the radioactive isotope C -14 differ from its stable C-14 = 6 p+ and 8 n counterpart C-12? o C-12 = 6 p+ and 6 no A. It has a different number of protons and two less neutrons than C-12. B. It has the same number of protons and two more electrons than C-12. C. It has the same number of protons but two more neutrons than C-12. D. It has a different number of protons and two more neutrons than C-12.

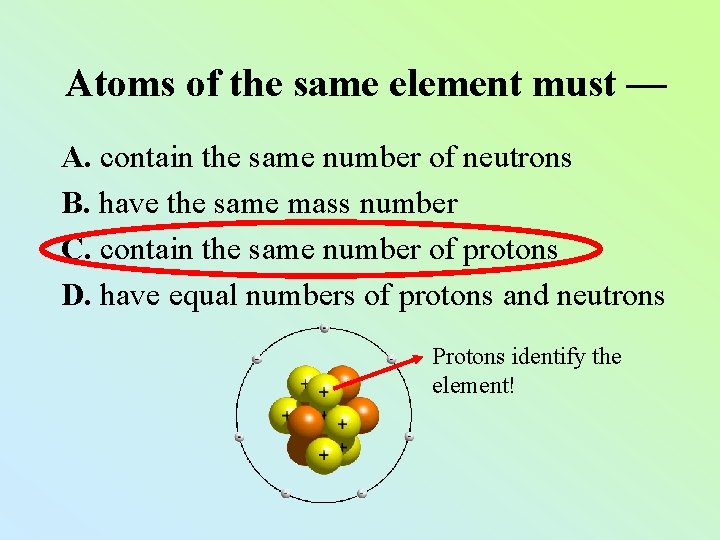
Atoms of the same element must — A. contain the same number of neutrons B. have the same mass number C. contain the same number of protons D. have equal numbers of protons and neutrons Protons identify the element!

The understanding that the position of an electron in an electron cloud cannot precisely be determined was developed by Werner I am uncertain Heisenberg and is known as the — where the e- is A. planetary model B. uncertainty principle C. quantum theory D. first atomic theory located!

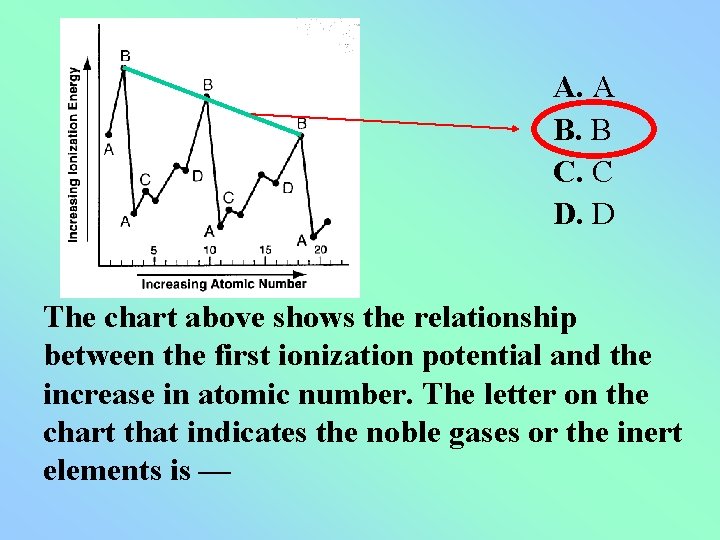
A. A B. B C. C D. D The chart above shows the relationship between the first ionization potential and the increase in atomic number. The letter on the chart that indicates the noble gases or the inert elements is —

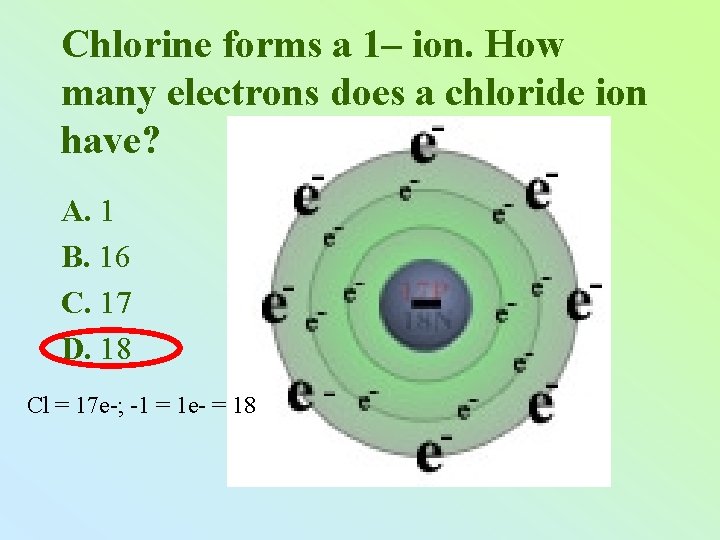
Chlorine forms a 1– ion. How many electrons does a chloride ion have? A. 1 B. 16 C. 17 D. 18 Cl = 17 e-; -1 = 1 e- = 18

Isotopes of an element have different — A. atomic numbers B. atomic masses C. numbers of protons D. numbers of outer-shell electrons

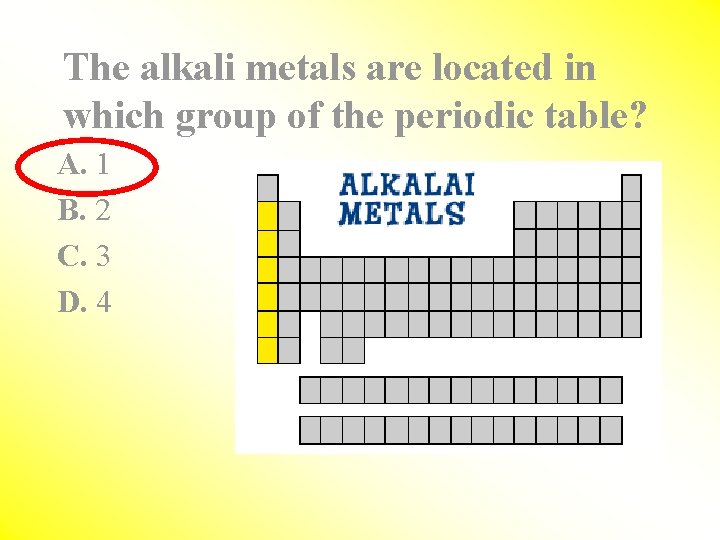
The alkali metals are located in which group of the periodic table? A. 1 B. 2 C. 3 D. 4

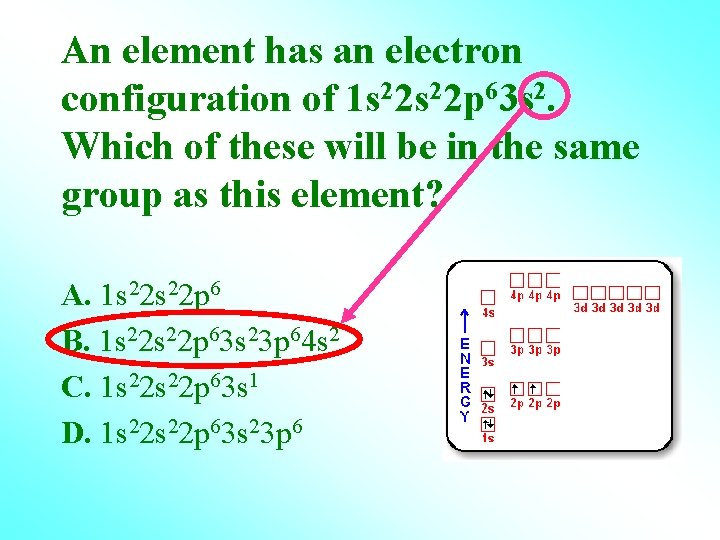
An element has an electron configuration of 1 s 22 p 63 s 2. Which of these will be in the same group as this element? A. 1 s 22 p 6 B. 1 s 22 p 63 s 23 p 64 s 2 C. 1 s 22 p 63 s 1 D. 1 s 22 p 63 s 23 p 6

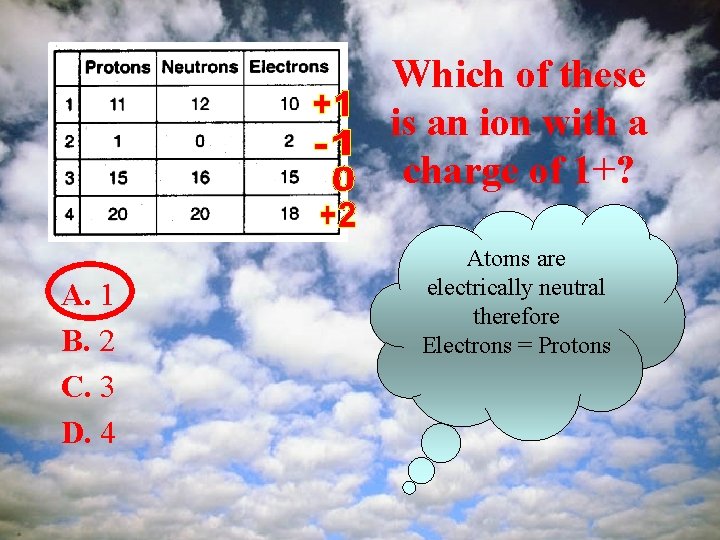
Which of these is an ion with a charge of 1+? A. 1 B. 2 C. 3 D. 4 Atoms are electrically neutral therefore Electrons = Protons

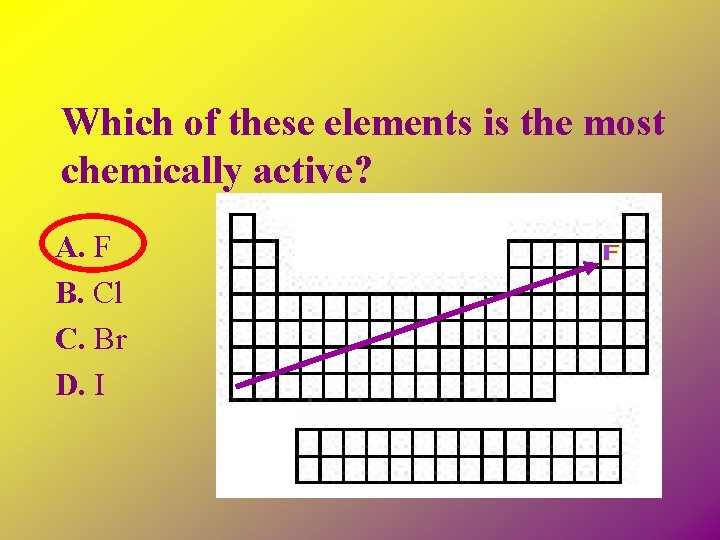
Which of these elements is the most chemically active? A. F B. Cl C. Br D. I

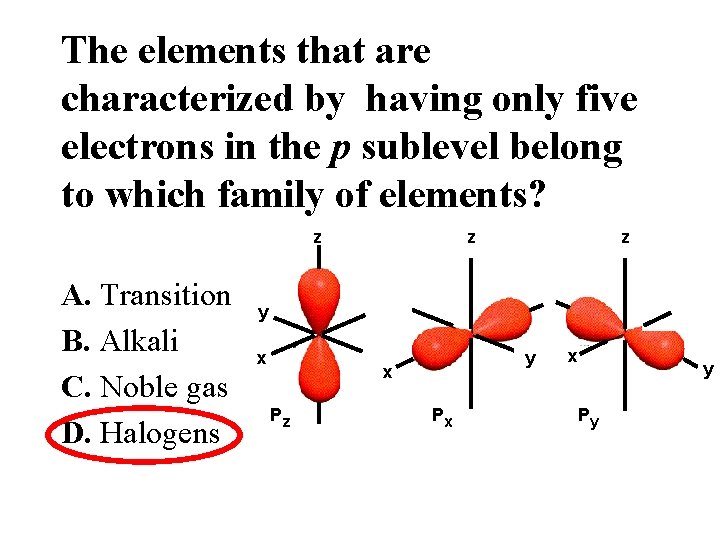
The elements that are characterized by having only five electrons in the p sublevel belong to which family of elements? z A. Transition B. Alkali C. Noble gas D. Halogens z z y x Pz Px x y Py

An increase in atomic number is related to an increase in atomic mass because — A. more electrons are present in the atomic nucleus B. more electrons are orbiting the atomic nucleus C. more protons are present in the atomic nucleus D. more protons are orbiting the atomic nucleus

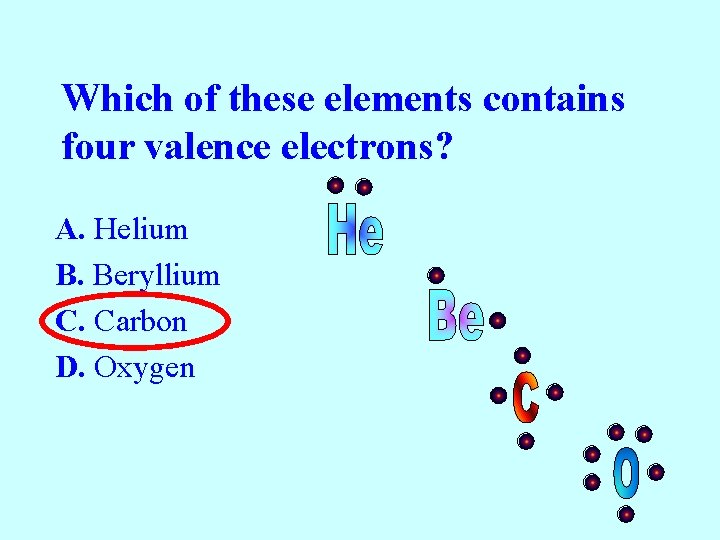
Which of these elements contains four valence electrons? A. Helium B. Beryllium C. Carbon D. Oxygen

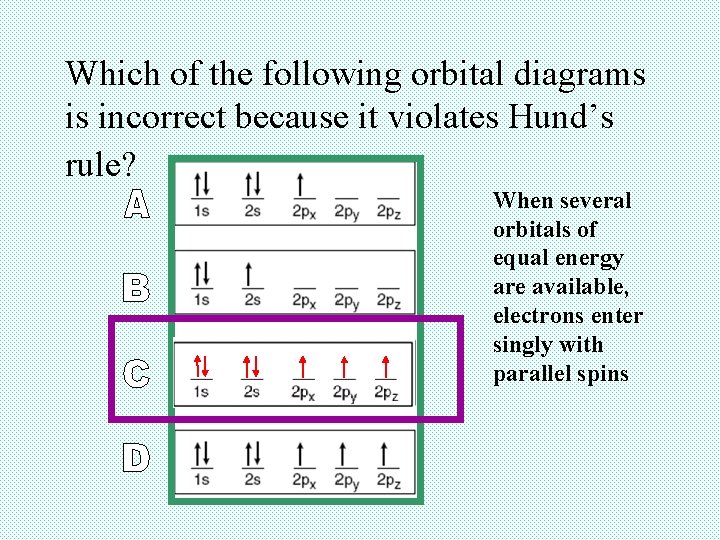
Which of the following orbital diagrams is incorrect because it violates Hund’s rule? When several orbitals of equal energy are available, electrons enter singly with parallel spins

What is the main similarity among elements in group 2? A. Atomic radius B. Chemical properties C. Mass number D. Boiling point

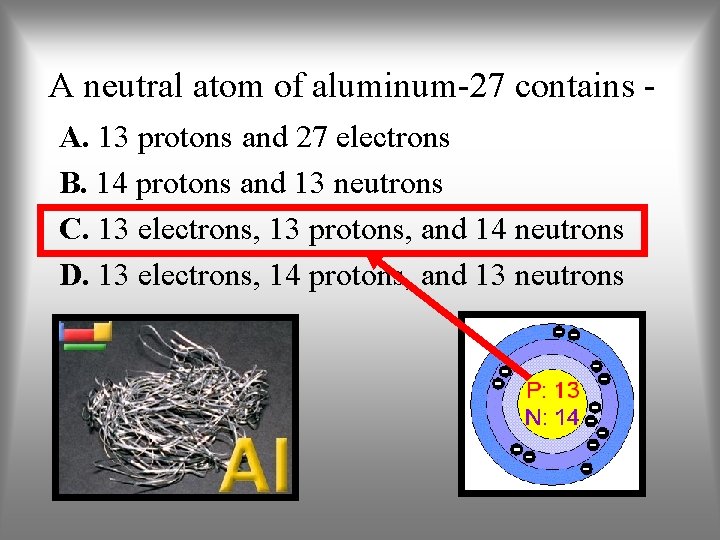
A neutral atom of aluminum-27 contains A. 13 protons and 27 electrons B. 14 protons and 13 neutrons C. 13 electrons, 13 protons, and 14 neutrons D. 13 electrons, 14 protons, and 13 neutrons

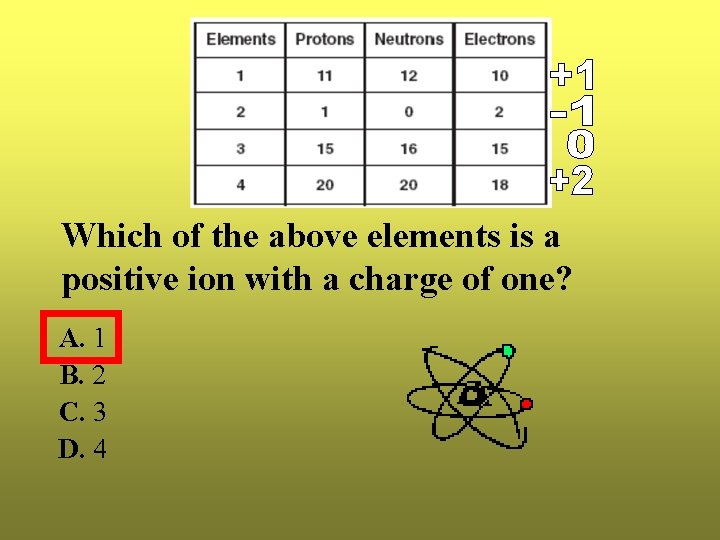
Which of the above elements is a positive ion with a charge of one? A. 1 B. 2 C. 3 D. 4

Cations are formed when neutral atoms lose — A. electrons B. protons C. neutrons D. positrons

Which of the following properties decreases from left to right across a period? A. Atomic number B. Electronegativity C. Atomic radius D. Ionization energy

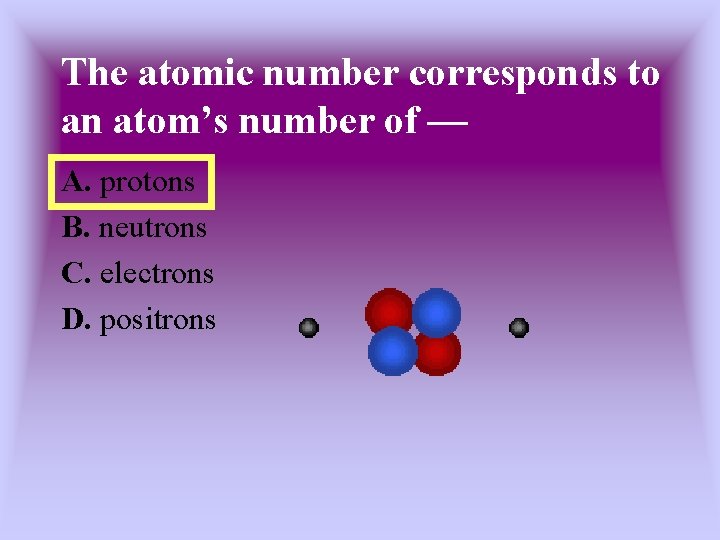
The atomic number corresponds to an atom’s number of — A. protons B. neutrons C. electrons D. positrons

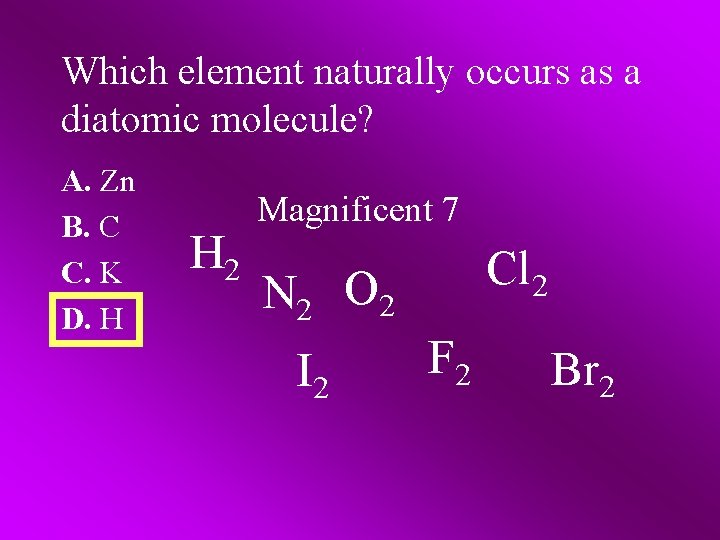
Which element naturally occurs as a diatomic molecule? A. Zn B. C C. K D. H H 2 Magnificent 7 Cl 2 N 2 O 2 I 2 F 2 Br 2

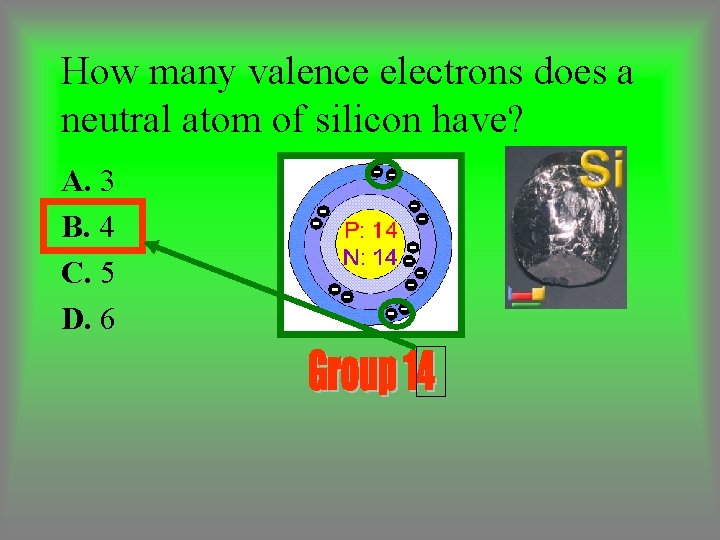
How many valence electrons does a neutral atom of silicon have? A. 3 B. 4 C. 5 D. 6

At room temperature, chlorine exists as a gas, bromine exists as a liquid, and iodine exists as a solid. The physical states of these elements indicate that melting point — A. decreases from top to bottom with group 17 elements B. is independent of periodic position C. increases from top to bottom within group 17 elements D. is constant within group 17 elements

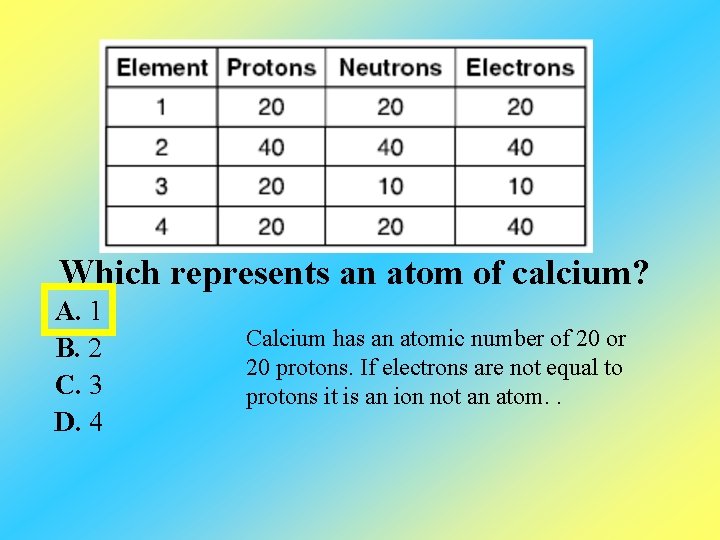
Which represents an atom of calcium? A. 1 B. 2 C. 3 D. 4 Calcium has an atomic number of 20 or 20 protons. If electrons are not equal to protons it is an ion not an atom. .
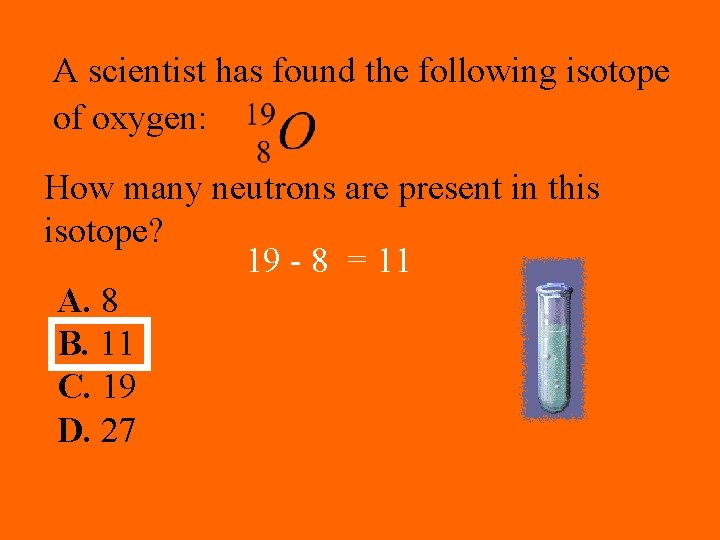
A scientist has found the following isotope of oxygen: How many neutrons are present in this isotope? 19 - 8 = 11 A. 8 B. 11 C. 19 D. 27

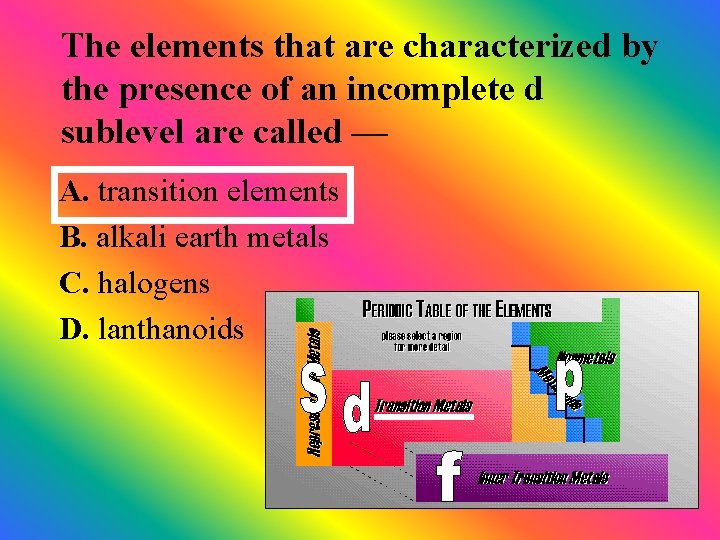
The elements that are characterized by the presence of an incomplete d sublevel are called — A. transition elements B. alkali earth metals C. halogens D. lanthanoids

The net charge on an aluminum ion is 3 because there are — A. 10 protons and 13 electrons in the atom B. 13 protons and 10 neutrons in the nucleus C. 10 neutrons and 13 electrons in the atom D. 13 protons and 10 electrons in the atom Al’s atomic number is 13 therefore it has 13 electrons and 13 protons but when it forms an ion it loses 3 electrons to achieve the configuration of a Nobel gas!

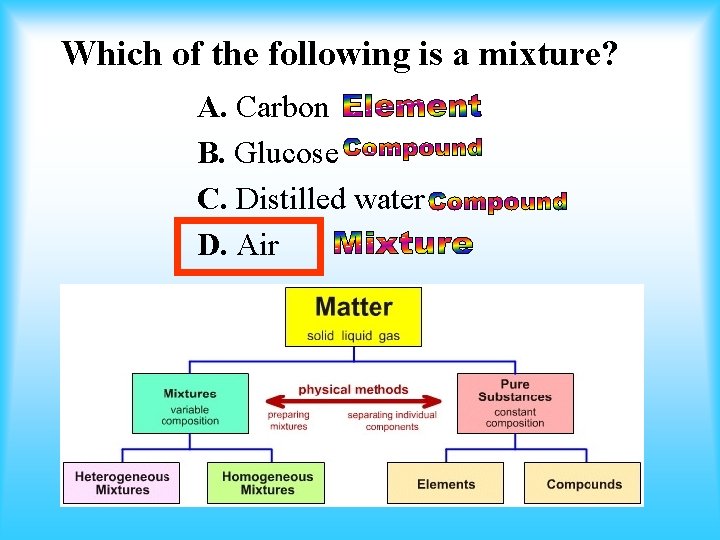
Which of the following is a mixture? A. Carbon B. Glucose C. Distilled water D. Air

One indicator that electrons in atoms are limited to specific energy levels is that — A. atoms move faster when heated B. the light given off by atoms is all at the same wavelength C. the Doppler effect shows a shift in wavelength for H-atom light D. light emitted from excited atoms occurs only at specific wavelengths

The End