Grade 12 Chemistry Knowledge area Chemical change Rate


![Rate of reaction Expressing the rate of reaction Quick facts Square brackets [ ] Rate of reaction Expressing the rate of reaction Quick facts Square brackets [ ]](https://slidetodoc.com/presentation_image_h2/ceca6367f8ec73b92760faf285b576f4/image-5.jpg)
![Rate of reaction -Δ[Reactants] Rate of reaction = Δt OR Δ[Products] Rate of reaction Rate of reaction -Δ[Reactants] Rate of reaction = Δt OR Δ[Products] Rate of reaction](https://slidetodoc.com/presentation_image_h2/ceca6367f8ec73b92760faf285b576f4/image-6.jpg)




- Slides: 18

Grade 12 Chemistry Knowledge area: Chemical change

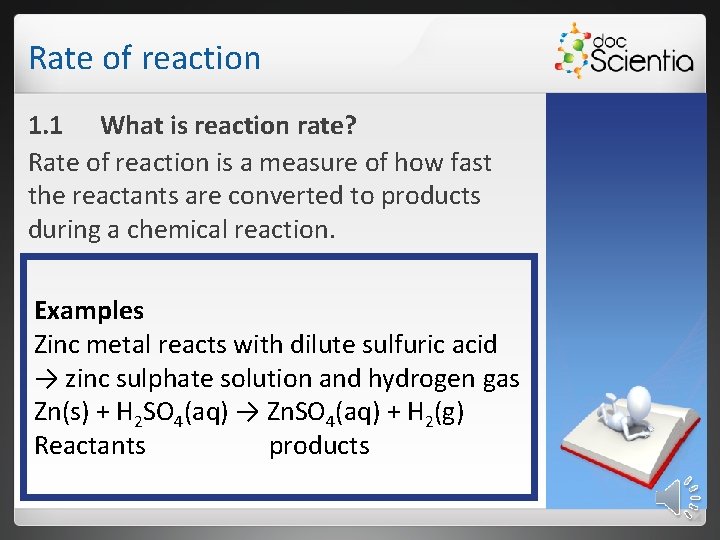
Rate of reaction 1. 1 What is reaction rate? Rate of reaction is a measure of how fast the reactants are converted to products during a chemical reaction. Examples Zinc metal reacts with dilute sulfuric acid → zinc sulphate solution and hydrogen gas Zn(s) + H 2 SO 4(aq) → Zn. SO 4(aq) + H 2(g) Reactants products

Rate of reaction The rate of this reaction can be measured by: • taking note of how fast the bubbles form; • measuring the time it takes for the zinc to decrease to nothing; • measuring the volume of H 2(g) that forms per time unit; • measuring the p. H of the solution if the excess acid is only just enough.

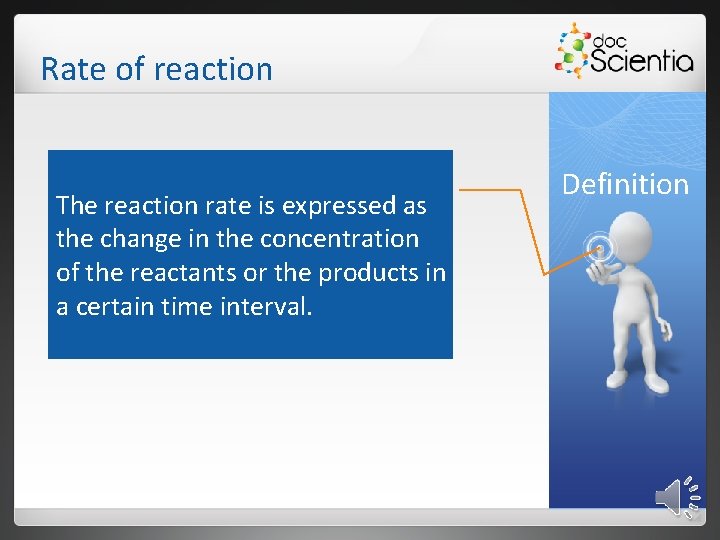
Rate of reaction The reaction rate is expressed as the change in the concentration of the reactants or the products in a certain time interval. Definition
![Rate of reaction Expressing the rate of reaction Quick facts Square brackets Rate of reaction Expressing the rate of reaction Quick facts Square brackets [ ]](https://slidetodoc.com/presentation_image_h2/ceca6367f8ec73b92760faf285b576f4/image-5.jpg)
Rate of reaction Expressing the rate of reaction Quick facts Square brackets [ ] are used to represent concentration. So, [H 2 SO 4(aq)] is the concentration of the sulfuric acid solution.
![Rate of reaction ΔReactants Rate of reaction Δt OR ΔProducts Rate of reaction Rate of reaction -Δ[Reactants] Rate of reaction = Δt OR Δ[Products] Rate of reaction](https://slidetodoc.com/presentation_image_h2/ceca6367f8ec73b92760faf285b576f4/image-6.jpg)
Rate of reaction -Δ[Reactants] Rate of reaction = Δt OR Δ[Products] Rate of reaction = Δt Quick facts The Δ[products] will have a positive value while the Δ[reactants] will have a negative value. The rate of reaction is always positive.

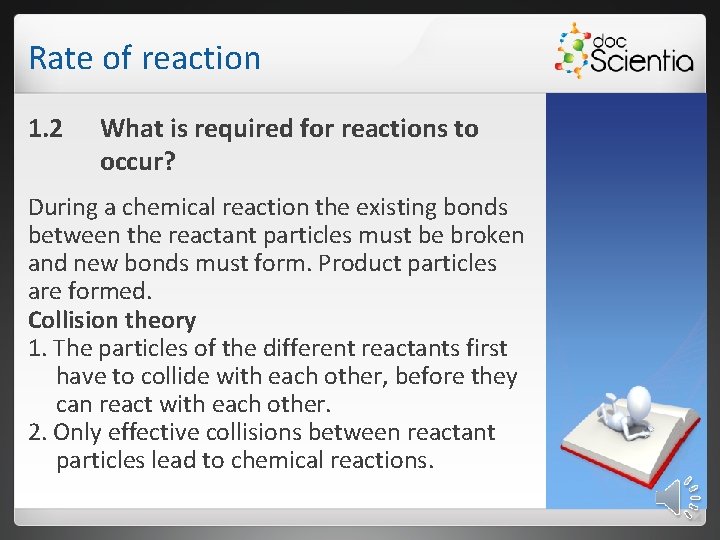
Rate of reaction 1. 2 What is required for reactions to occur? During a chemical reaction the existing bonds between the reactant particles must be broken and new bonds must form. Product particles are formed. Collision theory 1. The particles of the different reactants first have to collide with each other, before they can react with each other. 2. Only effective collisions between reactant particles lead to chemical reactions.

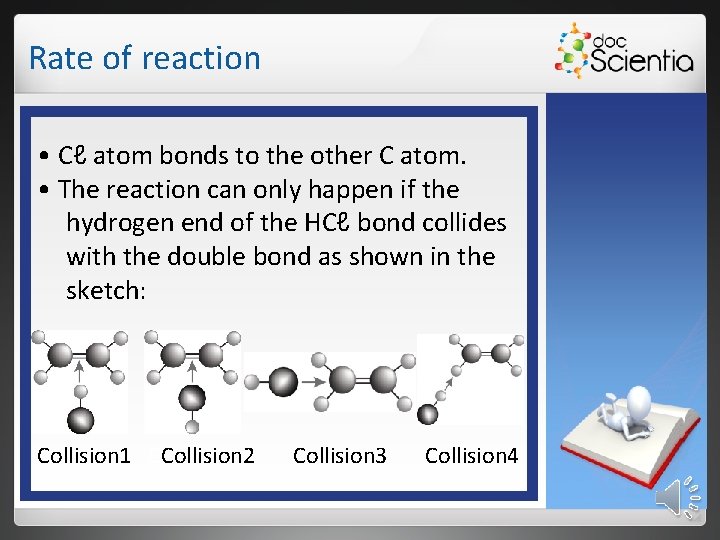
Rate of reaction For a collision to be effective: • the particles must have enough kinetic energy. • the particles must have the correct orientation. Examples CH 2=CH 2 + HCℓ → CH 3–CH 2–Cℓ • As a result of the collision between the two molecules, the double bond between the two C atoms is converted into a single bond. • H atom bonds to C atom.

Rate of reaction • Cℓ atom bonds to the other C atom. • The reaction can only happen if the hydrogen end of the HCℓ bond collides with the double bond as shown in the sketch: Collision 1 CCollision 2 Collision 3 Collision 4

Rate of reaction Particles in Collision 1 are in the correct orientation for an effective collision. If the particles have sufficient kinetic energy for an effective collision, it will lead to a reaction.

Rate of reaction 1. 3 Factors influencing reaction rates • Nature of the reactants • Concentration of the reactants and the pressure of gaseous reactants • Exposed reaction surface (state of division) of a solid reactant. • Temperature at which the reaction takes place. • Presence of a suitable catalyst

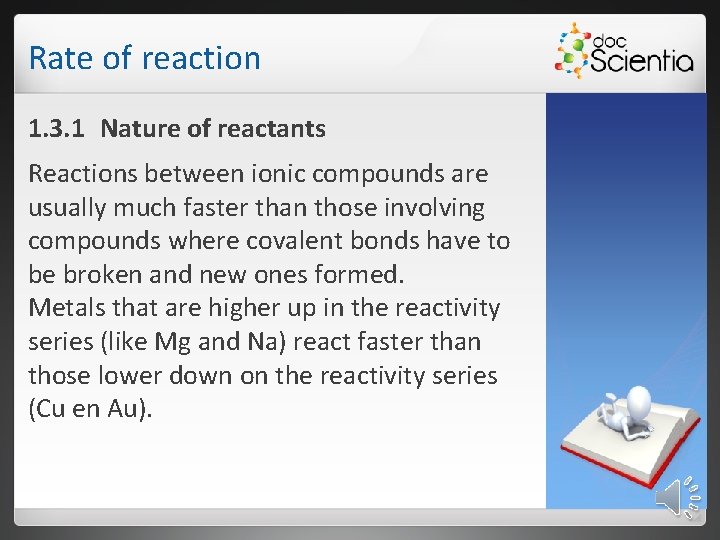
Rate of reaction 1. 3. 1 Nature of reactants Reactions between ionic compounds are usually much faster than those involving compounds where covalent bonds have to be broken and new ones formed. Metals that are higher up in the reactivity series (like Mg and Na) react faster than those lower down on the reactivity series (Cu en Au).

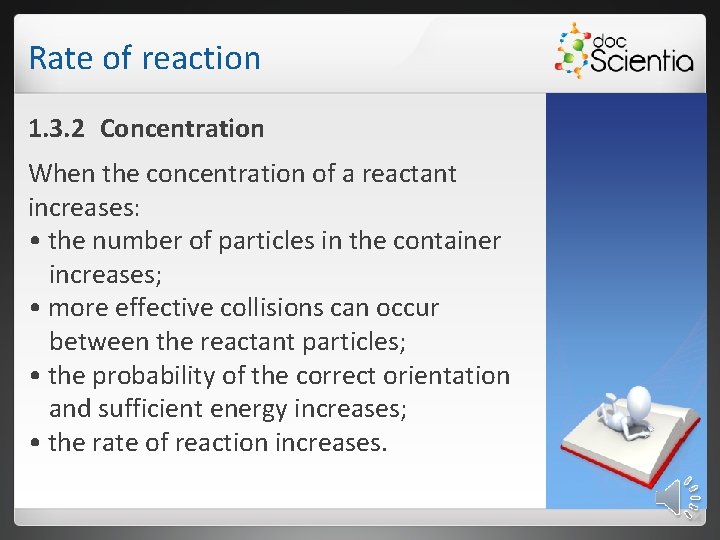
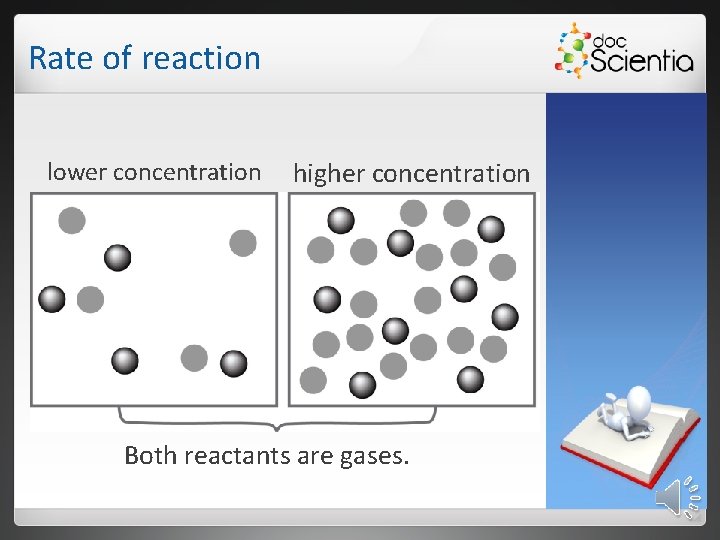
Rate of reaction 1. 3. 2 Concentration When the concentration of a reactant increases: • the number of particles in the container increases; • more effective collisions can occur between the reactant particles; • the probability of the correct orientation and sufficient energy increases; • the rate of reaction increases.

Rate of reaction lower concentration higher concentration Both reactants are gases.

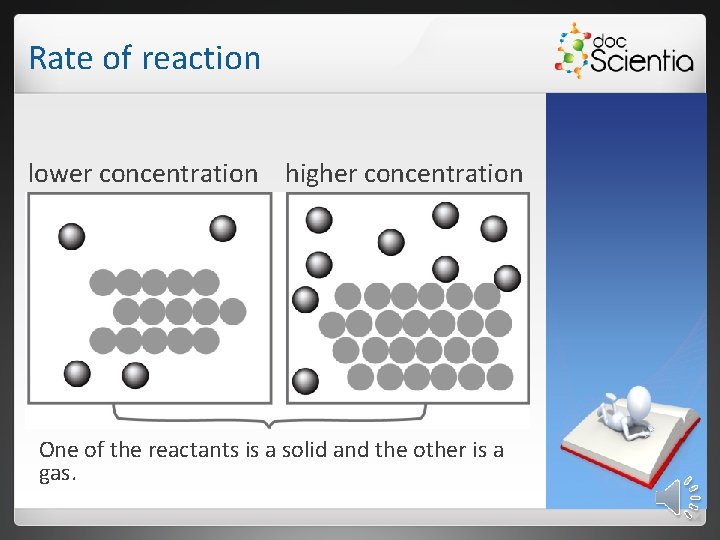
Rate of reaction lower concentration higher concentration One of the reactants is a solid and the other is a gas.

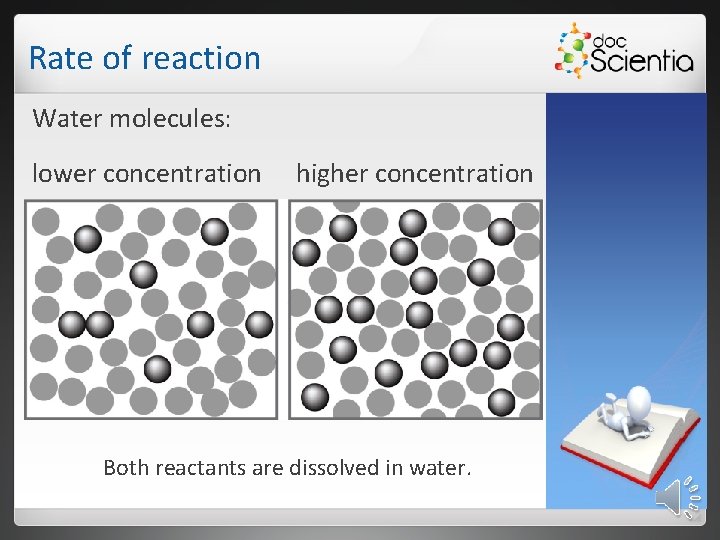
Rate of reaction Water molecules: lower concentration higher concentration Both reactants are dissolved in water.

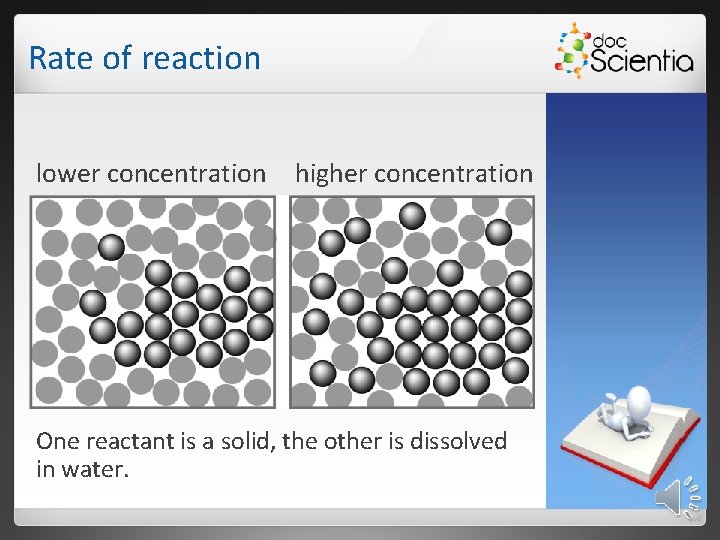
Rate of reaction lower concentration higher concentration One reactant is a solid, the other is dissolved in water.

Rate of reaction If the concentration of the reactants increases, the rate of the reaction will increase, since the number of effective collisions per second increases. Definition
 Chemistry grade 11 unit 4
Chemistry grade 11 unit 4 Is cutting paper a physical change
Is cutting paper a physical change Chemical and physical change
Chemical and physical change Difference between physical and chemical change
Difference between physical and chemical change Physical and chemical changes examples
Physical and chemical changes examples Spare change physical versus chemical change
Spare change physical versus chemical change Whats the difference between a physical and chemical change
Whats the difference between a physical and chemical change How does a physical change differ from a chemical change? *
How does a physical change differ from a chemical change? * What is an example of physical change
What is an example of physical change Is chopping firewood a physical or chemical change
Is chopping firewood a physical or chemical change Grade 11 quantitative aspects of chemical change
Grade 11 quantitative aspects of chemical change Chemical change grade 10
Chemical change grade 10 Personal vs shared knowledge
Personal vs shared knowledge Knowledge shared is knowledge squared
Knowledge shared is knowledge squared Knowledge shared is knowledge multiplied
Knowledge shared is knowledge multiplied Knowledge creation and knowledge architecture
Knowledge creation and knowledge architecture Contoh shallow knowledge dan deep knowledge
Contoh shallow knowledge dan deep knowledge A priori
A priori Book smarts vs street smarts
Book smarts vs street smarts