Grade 12 Chemistry Knowledge area Chemical change Rate


![Rate of reactions Exothermic reaction activated complex EP [A; 2 B] EA reactants A Rate of reactions Exothermic reaction activated complex EP [A; 2 B] EA reactants A](https://slidetodoc.com/presentation_image_h2/0a18d902ba936a5edaa7813587d7703a/image-9.jpg)

![Rate of reactions Endothermic reaction activated complex EP [P; 2 Q] EA reactants PQ Rate of reactions Endothermic reaction activated complex EP [P; 2 Q] EA reactants PQ](https://slidetodoc.com/presentation_image_h2/0a18d902ba936a5edaa7813587d7703a/image-12.jpg)
- Slides: 13

Grade 12 Chemistry Knowledge area: Chemical change

Rate of reactions 1. 6 Reaction mechanisms 1. 6. 1 Activation energy is the minimum energy required for a single reaction to occur between two or more particles. Definition

Rate of reactions Activation energy: • heat due to friction, like a match. • sunlight energy as in substitution reactions with some alkanes. • electrical energy as in neon lights, tube lights and other ionising gas lamps. • a single spark as in the combustion of fuel in vehicles. • mechanical energy is sometimes required to start a reaction, like stirring or shaking a mixture of reactants.

Rate of reactions 1. 6. 2 Activated complex Definition The activated complex is a temporary, high energy, unstable transitional state that exists for a short time (moments) between the products and the reactants.

Rate of reactions 1. 6. 3 Energy changes during chemical reactions We already know that the breaking of existing bonds requires an absorption of energy. The formation of new bonds leads to the release of energy. The difference in the amount of energy that is absorbed to break existing bonds and the energy that is released when new bonds form, is known as the change in enthalpy or heat of reaction.

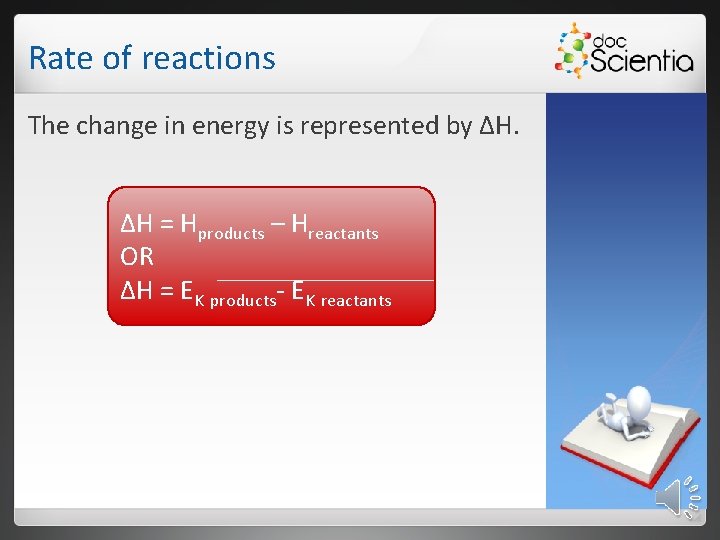
Rate of reactions The change in energy is represented by ΔH. ΔH = Hproducts – Hreactants OR ΔH = EK products- EK reactants

Rate of reactions Exothermic reaction Examples: Food being consumed. Any combustion reaction Acids that ionise in water. KOH/Na. OH that dissolve in water.

Rate of reactions Exothermic reaction • More energy is released when new bonds form than is absorbed to break existing bonds. • Temperature of reaction mixture rises. • ΔH < 0 (negative) • Reactants → products + energy • Reactants → products + k. J • Products have less potential energy than reactants. • Products are more stable than the reactants.
![Rate of reactions Exothermic reaction activated complex EP A 2 B EA reactants A Rate of reactions Exothermic reaction activated complex EP [A; 2 B] EA reactants A](https://slidetodoc.com/presentation_image_h2/0a18d902ba936a5edaa7813587d7703a/image-9.jpg)
Rate of reactions Exothermic reaction activated complex EP [A; 2 B] EA reactants A + B 2 ΔH < 0 enthalpy change products AB 2 time elapsed

Rate of reactions Endothermic reaction Examples: Photosynthesis KNO 3/NH 4 NO 3/Ba. Cℓ 2 that dissolve in water. Ice that melts.

Rate of reactions Endothermic reaction • More energy is absorbed to break existing bonds than is released when new bonds form. • Temperature of the reaction mixture decreases. • Δ H > 0 (positive) • Reactants + energy → products • Reactants + k. J → products • Products have more potential energy than reactants. • Products are less stable than the reactants.
![Rate of reactions Endothermic reaction activated complex EP P 2 Q EA reactants PQ Rate of reactions Endothermic reaction activated complex EP [P; 2 Q] EA reactants PQ](https://slidetodoc.com/presentation_image_h2/0a18d902ba936a5edaa7813587d7703a/image-12.jpg)
Rate of reactions Endothermic reaction activated complex EP [P; 2 Q] EA reactants PQ 2 ΔH < 0 enthalpy change time elapsed P + Q 2 products

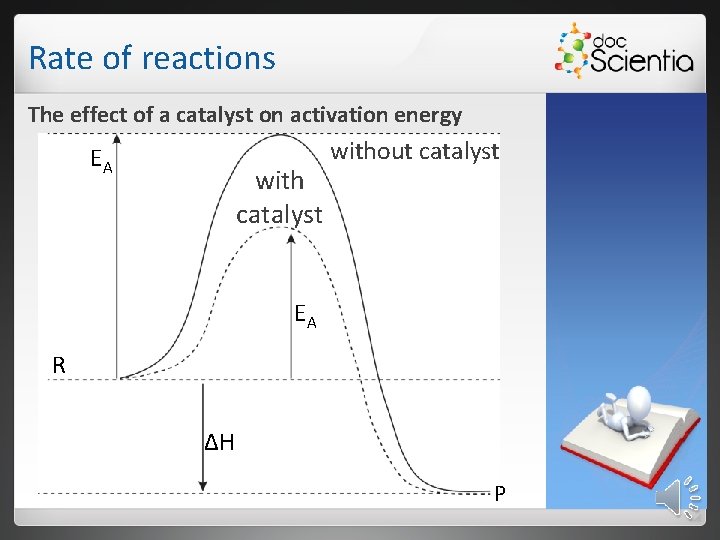
Rate of reactions The effect of a catalyst on activation energy EA with catalyst without catalyst EA R ΔH P
 Chemistry unit 4 grade 11
Chemistry unit 4 grade 11 Examples for physical change
Examples for physical change Chemical change vs physical change
Chemical change vs physical change Whats the difference between chemical and physical change
Whats the difference between chemical and physical change Physical vs chemical change examples
Physical vs chemical change examples Spare change physical versus chemical change
Spare change physical versus chemical change Whats the difference between a chemical and physical change
Whats the difference between a chemical and physical change Physical change
Physical change Chemical change in baking
Chemical change in baking Why is chopping wood a physical change
Why is chopping wood a physical change Quantitative aspects of chemical change grade 11
Quantitative aspects of chemical change grade 11 Physical and chemical change grade 10
Physical and chemical change grade 10 Shared knowledge vs personal knowledge
Shared knowledge vs personal knowledge Knowledge shared is knowledge squared meaning
Knowledge shared is knowledge squared meaning























