GMP issues in Q assessment Wondiyfraw Worku Assessor














- Slides: 17

GMP issues in Q assessment Wondiyfraw Worku Assessor 6 th CPH assessment training workshop, May 2014 1

Outline § Regulatory strategy- reminder § GMP documentations in Q dossier Ø Mfg license Ø GMP certificate Ø CPP § Dossier documentation that must be QA approved and signed § Assessor-inspector communication Ø Possible scenarios 2

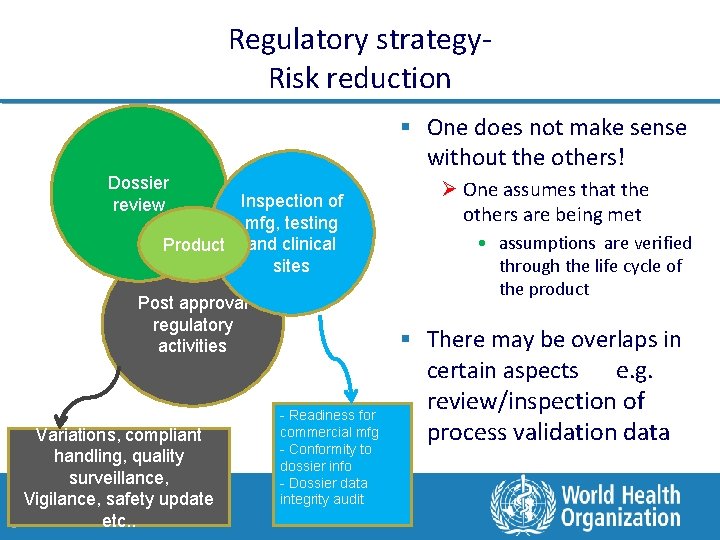
Regulatory strategy. Risk reduction § One does not make sense without the others! Dossier review Inspection of mfg, testing Product and clinical sites Post approval regulatory activities Variations, compliant handling, quality surveillance, Vigilance, safety update etc. . 3 - Readiness for commercial mfg - Conformity to dossier info - Dossier data integrity audit Ø One assumes that the others are being met • assumptions are verified through the life cycle of the product § There may be overlaps in certain aspects e. g. review/inspection of process validation data

GMP documentation in dossiers- provide minimum basis for review § Manufacturing license Øissued by national competent authority Øusually certifies that a given site has been authorized to perform the claimed duties Ømay or may not specify specific products but at least the authorized dosage form/line Øimportant to establish the basic legal and regulatory status of the manufacturer 4

GMP documentation- Contd § GMP certificate ØCertifies that a given site has been inspected by the national inspector (in accordance with local requirements) and deemed to be of acceptable compliance. • Local requirement may make reference to WHO GMP requirements but does not mean that the site has been inspected by WHO ØIn some jurisdictions, GMP certification is part of the manufacturing license 5

GMP documentation- Contd § Certificate of Pharmaceutical Product Ø WHO format, comprehensive Ø issued to a specific formulation Ø certifies whether the formulation has been reviewed/licensed by the country of origin and whether it is on the market Ø includes composition of the approved formulation Ø states all sites involved in manufacturing of the FPP but may not state API sites Ø may include summaries as approved by the issuing agency • Product information • Summary of basis of approval (similar to public assessment reports) 6

Certificate of Pharmaceutical Product-contd Ø Primarily intended to promote faster approval and speedy access to medicines by helping recipient countries to depend on sending authorities marketing authorization Ø Being also used in various other ways • As a key criteria for registration even though the application is supported by full dossier data • As supporting document for tenders • As a substitute for mfg license and GMP certificates Ø WHO’s blue book provides recommendations on appropriate use of CPP in various scenarios 7

WHO Blue Book recommendation 8

Implementation in PQ Full dossier route SRA route If available, CPP, should be submitted CPP as issued by the reference SRA - As a minimum, manufacturing license for should be submitted the specific sites involved in manufacturing activities should be submitted Registration or marketing in the country of origin is not a requirement - As we are fully reviewing and assuring the quality, safety/efficacy as well as compliance of CROs and manufacturing sites Registration and marketing in the country of origin is a requirement GMP certificate may be submitted to support the application - In any case, the site’s compliance status needs to be confirmed by our inspectors 9

Documents that must be signed by QA, dated and/or version tracked § Cover and undertaking letter § Application form- not applicable for PQ except variations and amendments § Letters of authorization § CEP/API PQ confirmation document § Filled and Blank BMRs § Process validation and stability protocols § Specifications § Commitments 10

Assessor-inspector communication § Via common databases Ø Easy traceability of all sites related to a given application § Via summary dossier information (QIS) Ø A quick but critical summary for inspection preparation § Assessment reports and inspection reports Ø e. g. summarized stability data with assessor’s interpretation Ø Inspection report from a previous inspection may tell compliance status of a given QC lab within a manufacturing site § Specific recommendations/feedbacks Ø Recommendation for inspection of raw data Ø The need for re-review of bioequivalence data § At the time of PQ Ø PQ decision form to be signed in by assessment and inspection heads 11

Examples of possible recommendations from assessors § Instances of unsolicited major changes in QA signed documentations (e. g. Specs, batch records) Ø Questions document approval procedure within the company § Instances of failure to investigate OOS or clear OOT results Ø Indicates a possible breach in quality system § Instances of “never completed” studies Ø e. g. stability studies that are claimed to have been interrupted 12

Examples- contd § Unaccounted deviations in batch records Ø Questions reliability of the batch record and the whole quality system § Failure to comply with written commitments Ø Seen during variation applications § Specific critical steps that may not be immediately visible in flow charts/method description but considered critical by assessors Ø e. g. steps that may involve or result in potential change in polymorph form Ø will help inspectors to factor in that in their inspection risk assessment 13

Example-contd § Inconsistent responses Ø e. g. - “ we have data” but then fails to provide the data Ø or they may state “ we do not have data” but then after a while “ we had the data” Ø “We did that” and after a while “ it was a typo” § Data that looks too good compared to prior experiences (“surprising results”) Ø for example, absence/ND levels of a major and common degradation product 14

Example-contd § Several versions of specifications and BMRs § Matters that may better be resolved by being at the site Ø e. g. issues related to media fill validation data • extent of simulation of interventions • extent of environmental control Ø e. g. release assay results close to limit, with no mass balance and with no other explanation 15

Example-contd § Very similar batch to batch results, as in the case of dissolution profiles § Too clean trend or too variable data § Unusual large number of rejects § Unusual large number of repeat analysis (BE) § Unaccounted protocol deviations (BE) § Significantly different p. K values compared to literature reported values (BE) 16

§ Thank you, Questions? 17
 Gmp assessor
Gmp assessor Anglia ruskin practice assessor
Anglia ruskin practice assessor Navajo county tax assessor
Navajo county tax assessor Chieta trade test
Chieta trade test Indlela trade centre
Indlela trade centre Auditor sqas assessor
Auditor sqas assessor Svq assessor
Svq assessor Shassic score
Shassic score Myeplg
Myeplg Polk county parcel map
Polk county parcel map Domestic energy assessor course near me
Domestic energy assessor course near me Paul d petersen
Paul d petersen Opassessor
Opassessor Lewis county wa assessor
Lewis county wa assessor Jim isbell
Jim isbell Assessor name
Assessor name Ndea training
Ndea training Rm assessor 3 ocr
Rm assessor 3 ocr

































