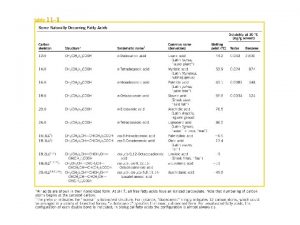
Glycerophospholipids and Lipoproteins Glycrophospholipids degradation Lipoproteins FATTY ACID





- Slides: 24

Glycerophospholipids and Lipoproteins Glycrophospholipids degradation Lipoproteins

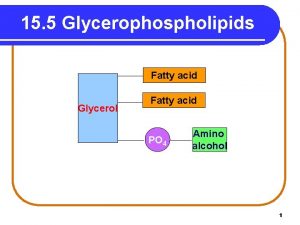
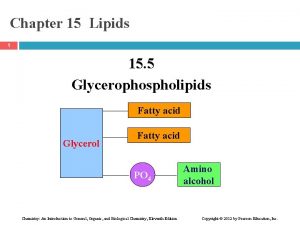
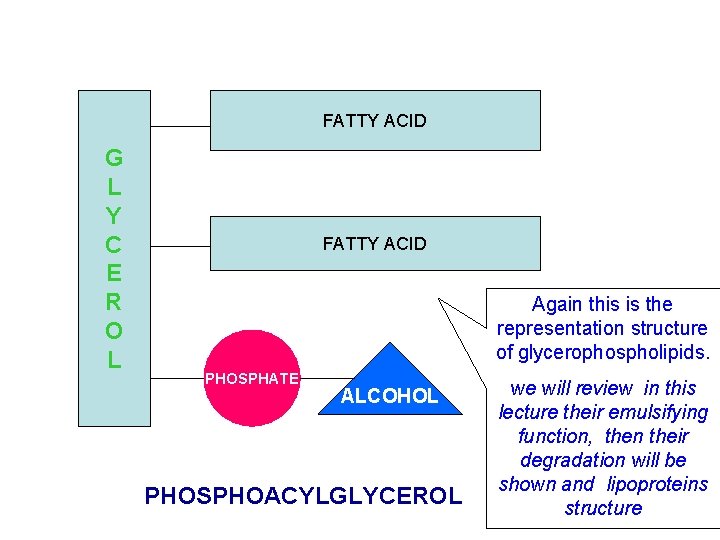
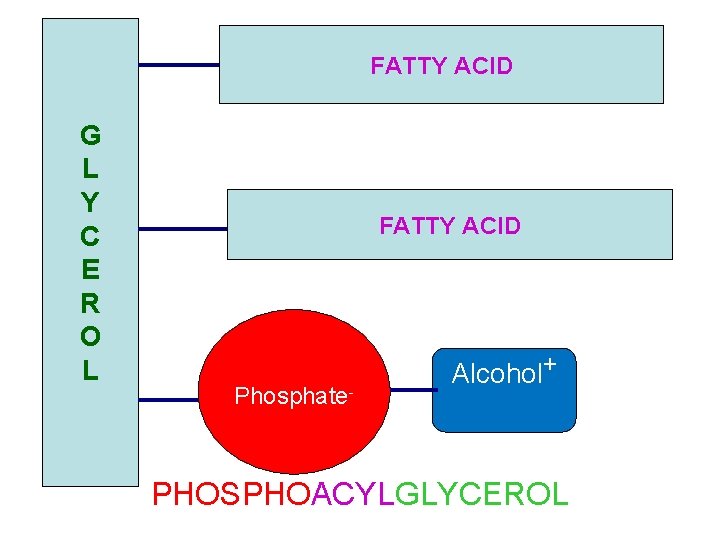
FATTY ACID G L Y C E R O L FATTY ACID Again this is the representation structure of glycerophospholipids. PHOSPHATE ALCOHOL PHOSPHOACYLGLYCEROL we will review in this lecture their emulsifying function, then their degradation will be shown and lipoproteins structure

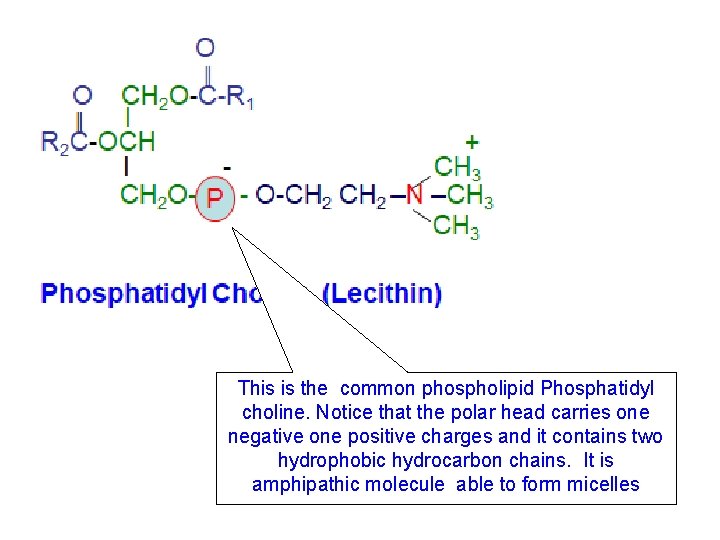
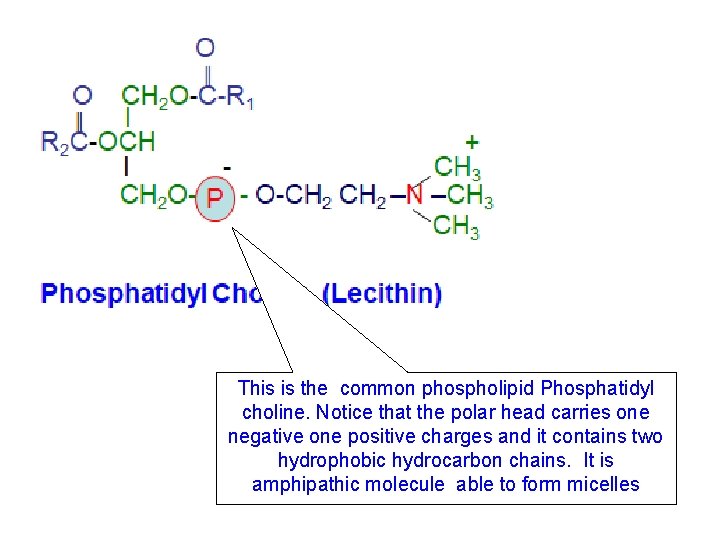
This is the common phospholipid Phosphatidyl choline. Notice that the polar head carries one negative one positive charges and it contains two hydrophobic hydrocarbon chains. It is amphipathic molecule able to form micelles

This is the common phospholipid Phosphatidyl choline. Notice that the polar head carries one negative one positive charges and it contains two hydrophobic hydrocarbon chains. It is amphipathic molecule able to form micelles

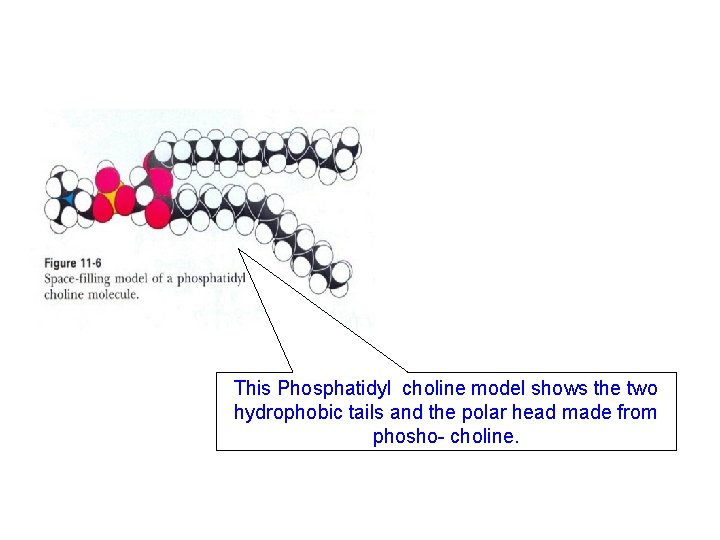
This Phosphatidyl choline model shows the two hydrophobic tails and the polar head made from phosho- choline.

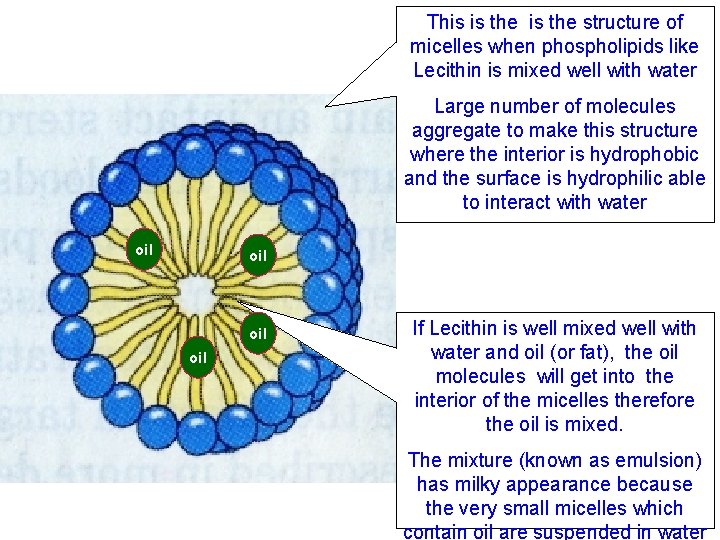
This is the structure of micelles when phospholipids like Lecithin is mixed well with water Large number of molecules aggregate to make this structure where the interior is hydrophobic and the surface is hydrophilic able to interact with water oil oil If Lecithin is well mixed well with water and oil (or fat), the oil molecules will get into the interior of the micelles therefore the oil is mixed. The mixture (known as emulsion) has milky appearance because the very small micelles which contain oil are suspended in water

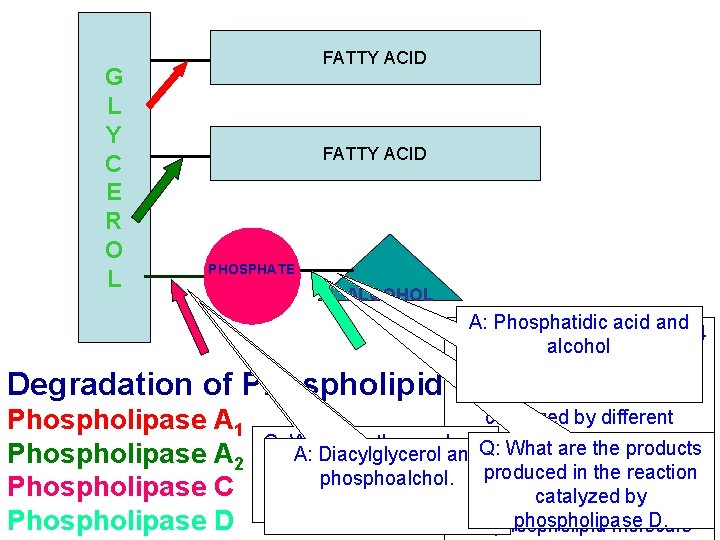
G L Y C E R O L FATTY ACID PHOSPHATE ALCOHOL A: Phosphatidic acid and Glycerophospholipids have 4 alcohol ester bonds. Degradation of Phospholipids: Hydrolysis of each bond is Phospholipase A 1 Phospholipase A 2 Phospholipase C Phospholipase D catalyzed by different enzymes. The are known as Q: What are the products A: Diacylglycerol and. Q: What are the products phospholipases. produced in the reaction phosphoalchol. catalyzed by Now seecatalyzed the actionby of each phospholipase C. phospholipase D. on phospholipid molecule

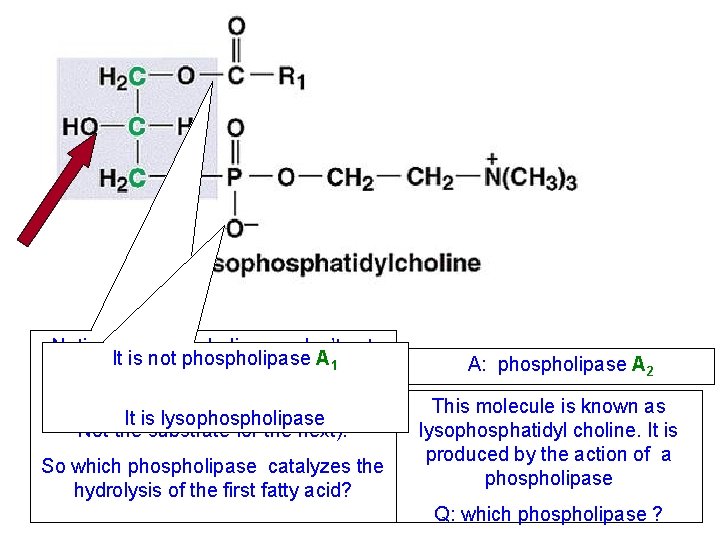
Notice that phospholipases don’t act It is not phospholipase A 1 sequentially (The product of one phospholipase is It is lysophospholipase Not the substrate for the next). So which phospholipase catalyzes the hydrolysis of the first fatty acid? A: phospholipase A 2 This molecule is known as lysophosphatidyl choline. It is produced by the action of a phospholipase Q: which phospholipase ?



Transport of TAG by Plasma Lipoproteins

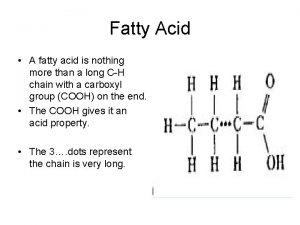
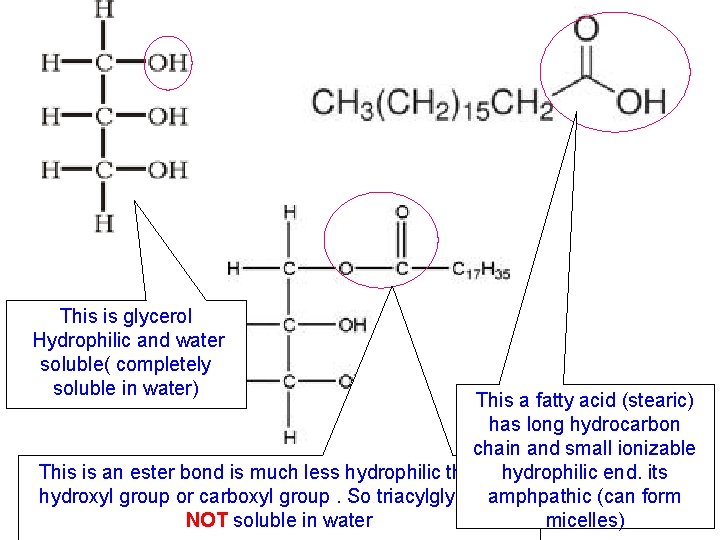
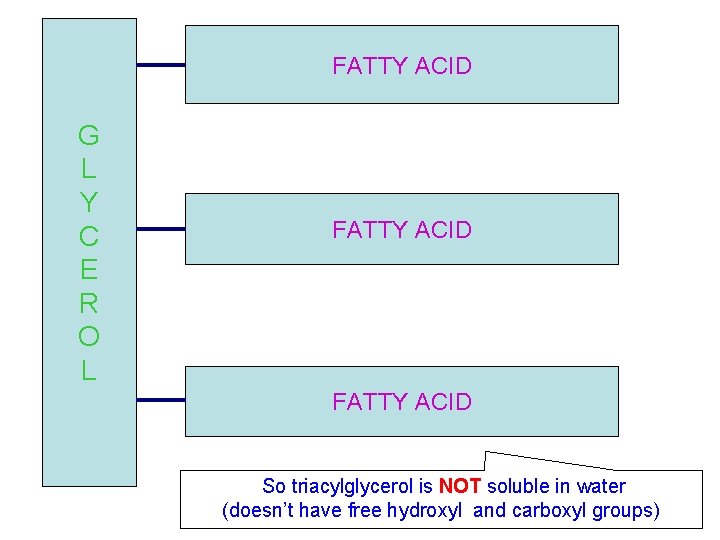
This is glycerol Hydrophilic and water soluble( completely soluble in water) This a fatty acid (stearic) has long hydrocarbon chain and small ionizable This is an ester bond is much less hydrophilic than the hydrophilic end. its hydroxyl group or carboxyl group. So triacylglycerol is amphpathic (can form NOT soluble in water micelles)

FATTY ACID G L Y C E R O L FATTY ACID So triacylglycerol is NOT soluble in water TRIACYLGLYCEROL (doesn’t have free hydroxyl and carboxyl groups)

FATTY ACID G L Y C E R O L FATTY ACID Phosphate- Alcohol+ PHOSPHOACYLGLYCEROL

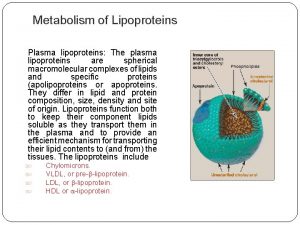
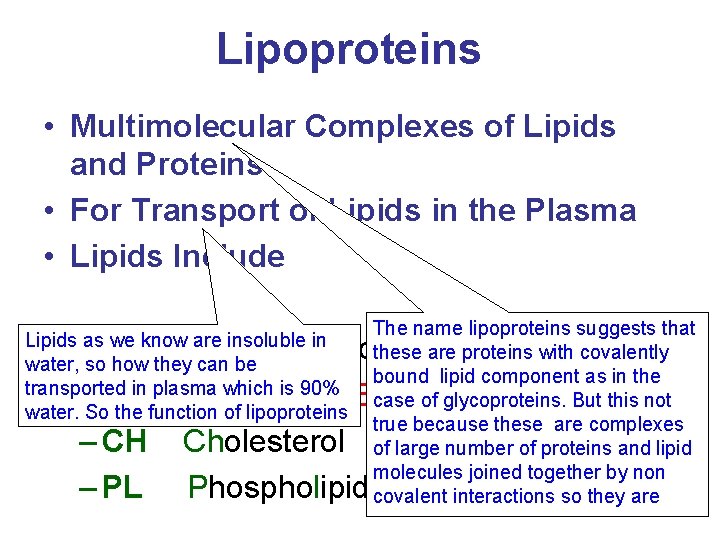
Lipoproteins • Multimolecular Complexes of Lipids and Proteins • For Transport of Lipids in the Plasma • Lipids Include Lipids as we know are insoluble in water, so how they can be transported in plasma which is 90% water. So the function of lipoproteins The name lipoproteins suggests that these are proteins with covalently bound lipid component as in the case of glycoproteins. But this not true because these are complexes of large number of proteins and lipid molecules joined together by non covalent interactions so they are – TAG Triacylglycerol – CE Cholesterol Ester – CH Cholesterol – PL Phospholipids

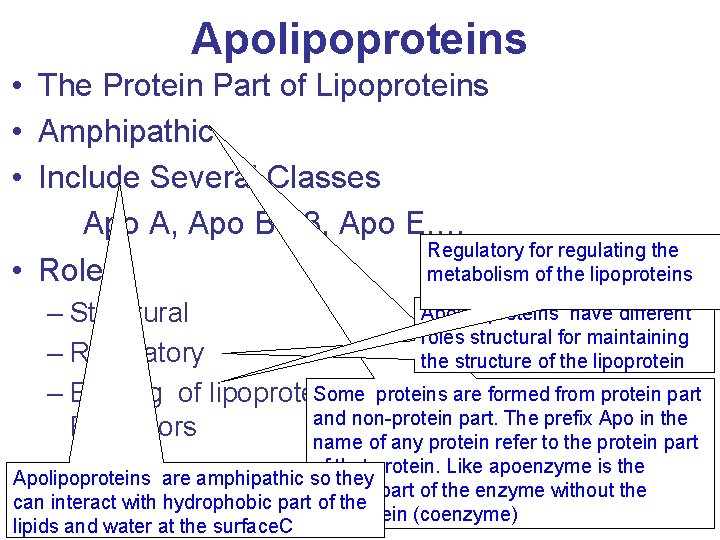
Apolipoproteins • The Protein Part of Lipoproteins • Amphipathic • Include Several Classes Apo A, Apo B-48, Apo E. … Regulatory for regulating the • Roles metabolism of the lipoproteins Apolipoproteins have different – Structural roles structural for maintaining – Regulatory the structure of the lipoprotein Sometoproteins are formed from protein part – Binding of lipoproteins Cell Surface and non-protein part. The prefix Apo in the Receptors name of any protein refer to the protein part of that protein. Like apoenzyme is the Apolipoproteins are amphipathic so they protein part of the enzyme without the can interact with hydrophobic part of the nonprotein (coenzyme) lipids and water at the surface. C

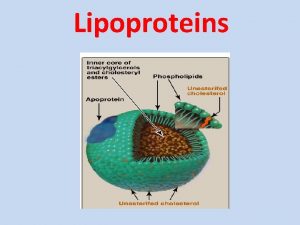
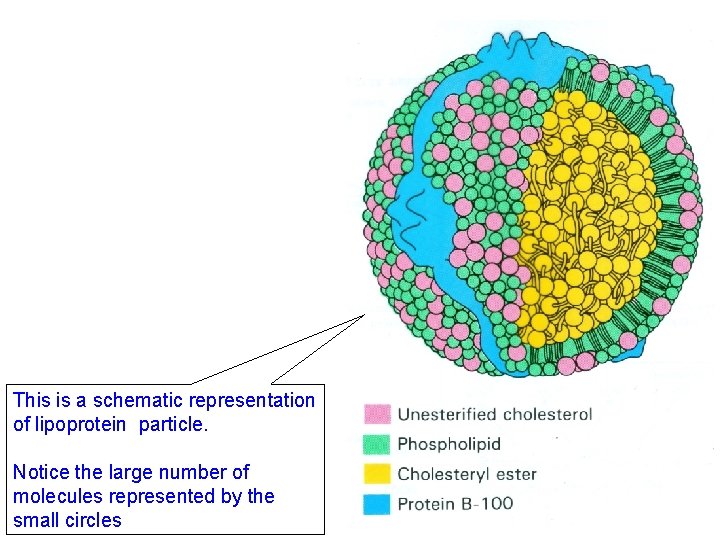
This is a schematic representation of lipoprotein particle. Notice the large number of molecules represented by the small circles

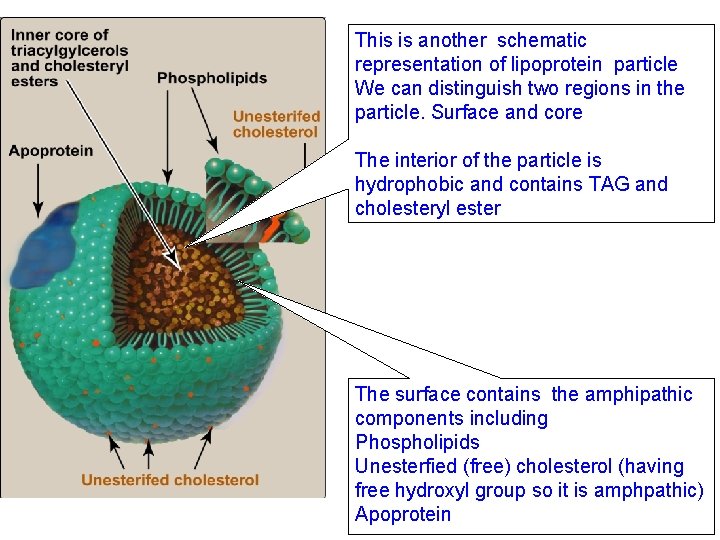
This is another schematic representation of lipoprotein particle We can distinguish two regions in the particle. Surface and core The interior of the particle is hydrophobic and contains TAG and cholesteryl ester The surface contains the amphipathic components including Phospholipids Unesterfied (free) cholesterol (having free hydroxyl group so it is amphpathic) Apoprotein

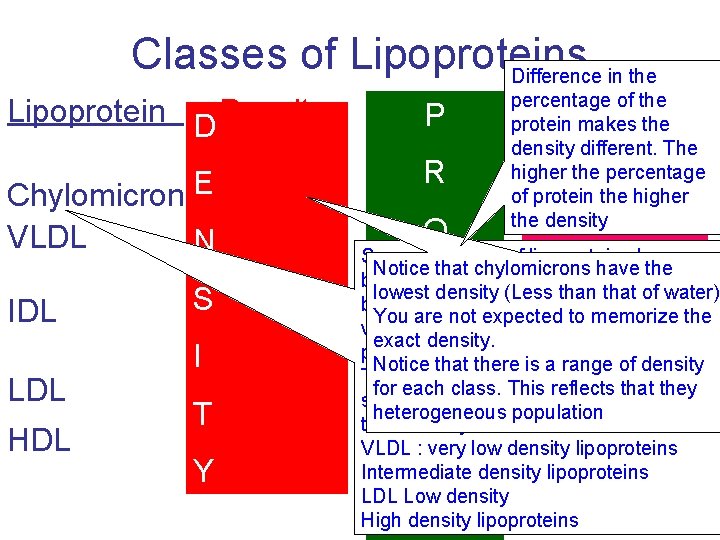
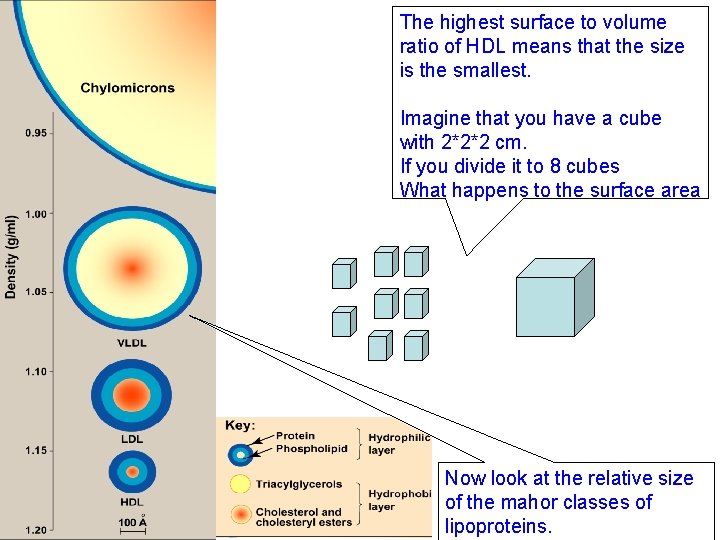
Classes of Lipoproteins Difference in the Lipoprotein D Density Protein P Chylomicrons. E <0. 95 VLDL N 0. 95 - 1. 006 R IDL LDL HDL S 1. 006 -1. 019 I 1. 019 - 1. 063 T 1. 063 - 1. 21 Y 2% percentage of the Majo Lipid protein makes the density different. The higher the percentage of protein higher TAGthe (85%) the density Major Lipid L I TAG (55%) Several classes of lipoproteins have Notice that chylomicrons have the been identified which be separated P can lowest. Tdensity (Less than that of water) O 9% by a technique called ultracentrifigation, (26%)the You 11% are not expected. TAG to memorize very high speed centriguge that exact density. CE (30%) produces very high g force. Notice that there is a range of density The lipoproteins particles will be for each This CE reflects(35%) that they 20%class. separated based in the difference in heterogeneous population their density PLlipoproteins (25%) VLDL 45% : very low density Intermediate density lipoproteins LDL Low density High density lipoproteins E I I D N S %

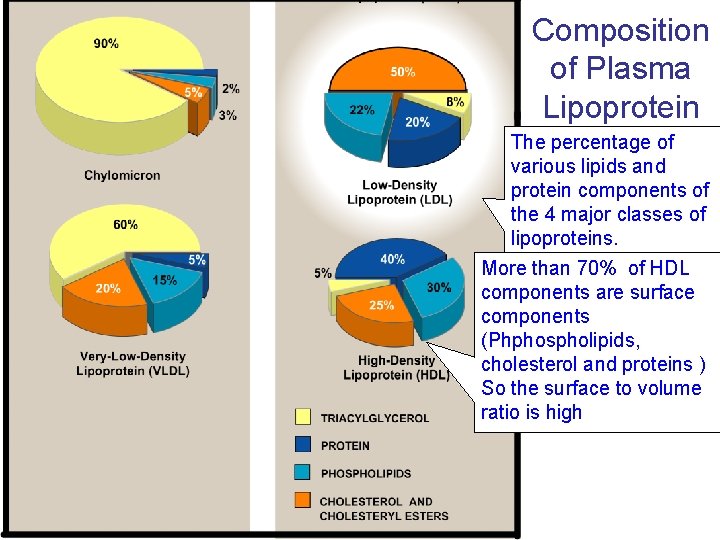
Composition of Plasma Lipoprotein The percentage of various lipids and protein components of the 4 major classes of lipoproteins. More than 70% of HDL components are surface components (Phphospholipids, cholesterol and proteins ) So the surface to volume ratio is high

The highest surface to volume ratio of HDL means that the size is the smallest. Imagine that you have a cube with 2*2*2 cm. If you divide it to 8 cubes What happens to the surface area Now look at the relative size of the mahor classes of lipoproteins.

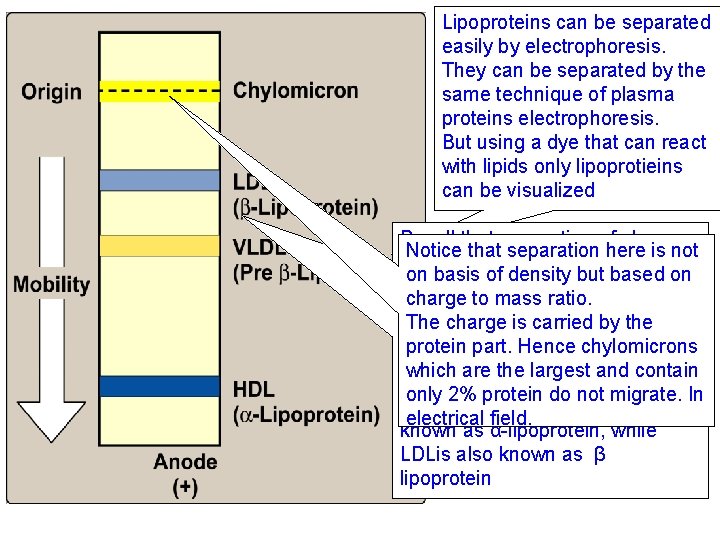
Lipoproteins can be separated easily by electrophoresis. They can be separated by the same technique of plasma proteins electrophoresis. But using a dye that can react with lipids only lipoprotieins can be visualized Recall that separation of plasma Notice that separation here is not proteins produces the following on basis of density but based on fractions, α 1, α 2, β and γ. So charge to mass ratio. plasma lipoproteins are also The charge is carried by the identified by the corresponding protein part. Hence chylomicrons plasma protein with which they which are the largest and contain comigrate. only 2% protein do not migrate. In Hence HDL for example is also electrical field. known as α-lipoprotein, while LDLis also known as β lipoprotein

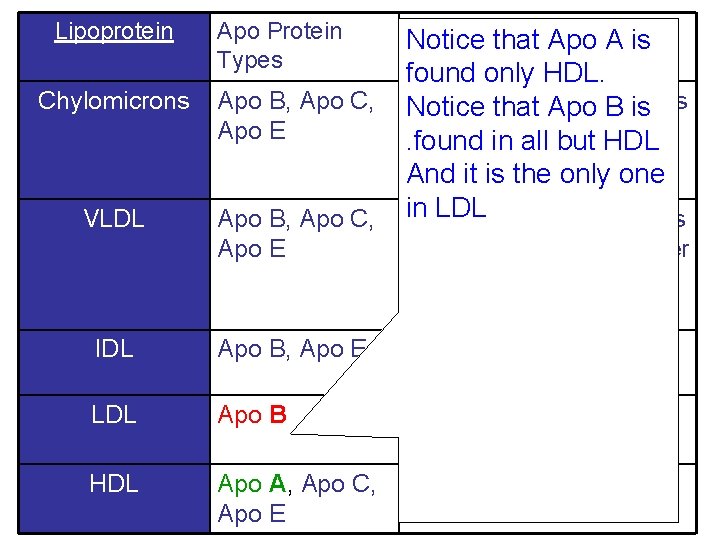
Lipoprotein Apo Protein Types Chylomicrons Apo B, Apo C, Apo E VLDL Apo B, Apo C, Apo E Functionthat Apo A is Notice found only HDL. Transport of Apo dietary Notice that B lipids is from small intestine to. found in all but HDL tissues And it is the only one in LDL of endogenous Transport TAG produced in the liver to tissues IDL Apo B, Apo E LDL Apo B Transfer of Cholesterol to tissue HDL Apo A, Apo C, Apo E Cholesterol Return to Liver

Next Topic is digestion of lipids
 Liposomes vs micelles
Liposomes vs micelles Triacontanoylpalmitate
Triacontanoylpalmitate Plfa soil test
Plfa soil test Fatty acid synthesis
Fatty acid synthesis Iupac name of fatty acid
Iupac name of fatty acid Triacylglycerols
Triacylglycerols Fatty acid synthesis
Fatty acid synthesis Fatty acid oxidation
Fatty acid oxidation Fatty acid synthesis steps
Fatty acid synthesis steps A saturated fatty acid holds all the hydrogen atoms it can.
A saturated fatty acid holds all the hydrogen atoms it can. Chemical properties of fatty acid
Chemical properties of fatty acid Pufas examples
Pufas examples Fatty acid composed of
Fatty acid composed of Function of lipids
Function of lipids What is essential fatty acid
What is essential fatty acid Beta oxidation of fatty acids
Beta oxidation of fatty acids Define saturated fatty acid
Define saturated fatty acid Fat deficiency
Fat deficiency Proteolipids vs lipoprotein
Proteolipids vs lipoprotein Lipoproteins in biochemistry
Lipoproteins in biochemistry Lipoproteins
Lipoproteins Lipoproteins
Lipoproteins Lipoproteins
Lipoproteins Lipoproteins
Lipoproteins Functions of lipoproteins
Functions of lipoproteins