Glass Properties and Analysis Glass Fragments Glass hard


















- Slides: 26

Glass Properties and Analysis

Glass Fragments � Glass: hard, brittle, amorphous substance that is composed of silicon oxide (sand) and other various metal oxides � Refraction—Bending of a light wave as it passes through one medium to another

Amorphous Solids �Amorphous solids: atoms or molecules are arranged RANDOMLY �NO regular order to the atoms ◦ Glass

Typical Types of Glass � Pyrex: A borosilicate— boron oxide is added to the glass to increase resistance to heat � Tempered Glass: glass to which strength is added by introducing stress through rapid heating and cooling of the glass surfaces ◦ When tempered glass breaks, it does not shatter ◦ Usually found in car windows because of safety issues Laminated Glass: two sheets of ordinary glass bonded together with plastic film ◦ Usually used in auto windshields in the United States

Glass Comparison � Comparison of glass is possible with broken or fractured glass through: ◦ 1. Glass Properties: ◦ ◦ �Density �Refractive Index (Becke Line) 2. 3. 4. 5. Physical Match Probability of common origin Direction of Impact Ballistics Sequence of Impact

Physical Property: Density �A physical property of matter that is equivalent to the mass-per-unit volume of a substance � Density: D= m/v (mass= grams, volume=m. L)


Density � Density is an INTENSIVE PROPERTY of matter, meaning it is the same regardless of the size of a substance and can be used as an aid in identification. � Solids tend to be more dense than liquids, and liquids more dense than gases.

Density � It is important to keep controlled temperatures when calculating Density. The volumes of gases and liquids vary considerably with temperatures. When the temperature of water increases it will expand, causing a difference in Density.

Density � Example: ◦ Water at 4℃ Density = 1. 0 g/m. L ◦ Water at 20℃ Density = 0. 998 g/m. L

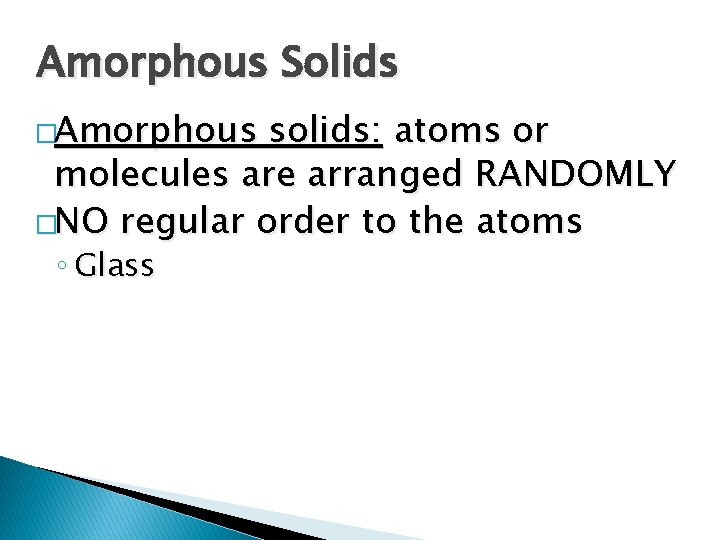
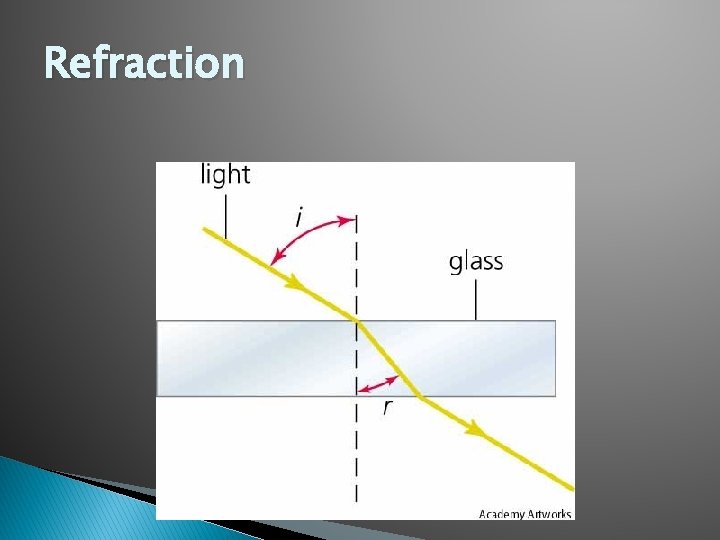
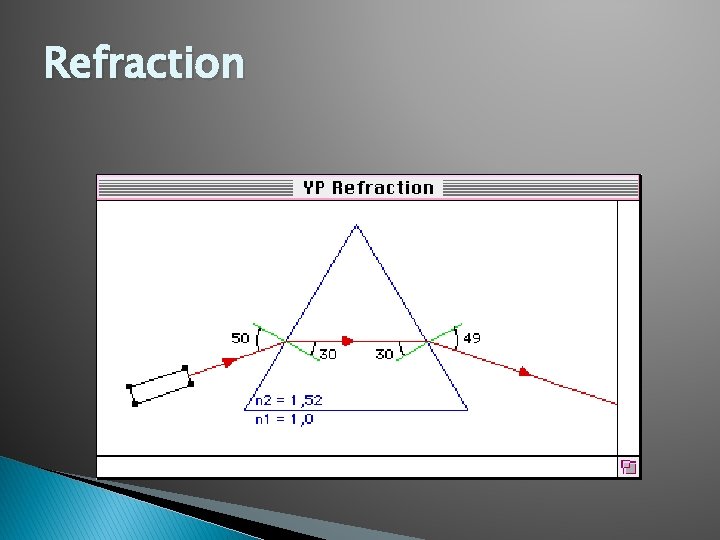
Refraction � Refraction: The bending of a light wave as it passes from one medium to another.

Refraction

Refraction

Refraction

Refraction

Refraction

Refraction � Glass that is broken and shattered into fragments during a crime can be used to place a suspect at the crime scene. � Example: During a burglary, glass can be lodged in a suspects shoe or garment. Also, headlight glass found at a hit-and-run scene may offer clues that can confirm the identity of the suspect vehicle.

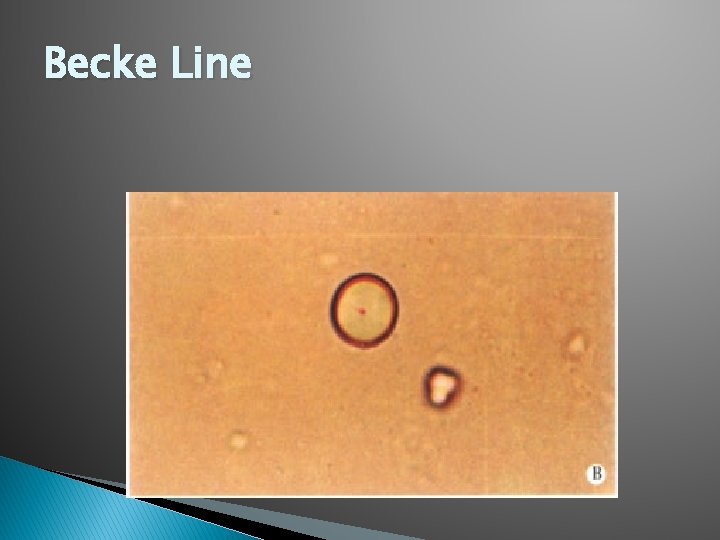
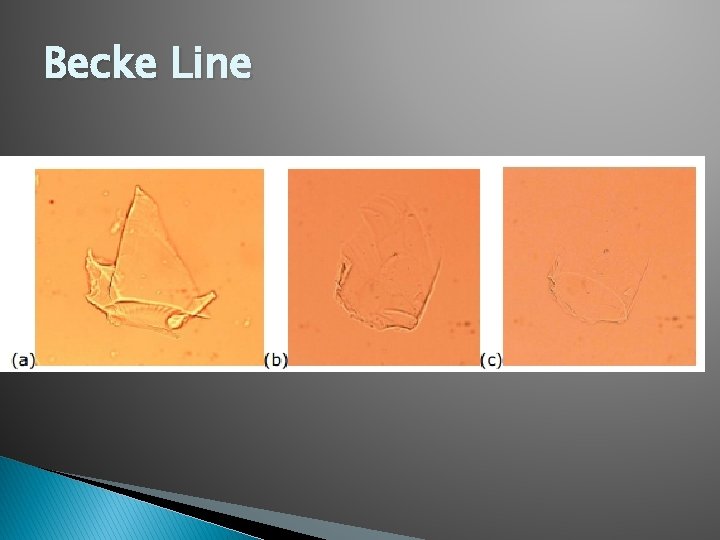
Becke Line �A bright halo that is observed near the border of a particle immersed in a liquid of a different refractive index.

Becke Line

Becke Line

Becke line � In other words: ◦ You will see the edges of the particle (glass) underneath a microscope.

Becke Line

Becke Line � Match Point- the point where the Becke Line is no longer observable. At this point you have reached the particles Refractive Index. You are also able to compare an unknown to a known.

Becke Line

Refraction � To determine the Refractive index of a piece of glass, one utilizes the Immersion Method. For this, glass particles are immersed in a liquid medium whose refractive index is varied until it is equal to that of the glass particles. At this point, known as the Match Point, the observer will note the disappearance of the Becke Line and minimum contrast between the glass and liquid medium.

Glass Comparison � Difficult because of prevalence of glass in our society � Often matched by piecing fragments together like a puzzle
 What makes water hard
What makes water hard Work hard play hard make history
Work hard play hard make history Hard times hard drive
Hard times hard drive Sentence fragments and run-on sentences
Sentence fragments and run-on sentences Identifying and correcting fragments
Identifying and correcting fragments Avoiding fragments and run-ons
Avoiding fragments and run-ons What are sentence fragment
What are sentence fragment Awubist
Awubist The nation and its fragments
The nation and its fragments Correcting fragments
Correcting fragments Example of hard glass
Example of hard glass Extensive vs intensive
Extensive vs intensive Chemical and physical properties
Chemical and physical properties Between a rock and a hard place analysis
Between a rock and a hard place analysis Platelets are fragments of multinucleate cells called
Platelets are fragments of multinucleate cells called Sentence fragment powerpoint
Sentence fragment powerpoint Mass spec fragments
Mass spec fragments Identifying sentence fragments
Identifying sentence fragments Dna fragments can be separated by
Dna fragments can be separated by Okazaki fragments founder
Okazaki fragments founder Dna fragments can be separated by
Dna fragments can be separated by Fragments in sentences
Fragments in sentences Types of sentence fragments
Types of sentence fragments Dfd fragments
Dfd fragments Dfd fragment
Dfd fragment Dfd fragments
Dfd fragments Platre de graffin
Platre de graffin