Ganymede the largest moon in the Solar System













- Slides: 45

Ganymede, the largest moon in the Solar System (larger than Mercury and Pluto), has a surface speckled with bright young craters overlying a mixture of older, darker, and more cratered terrain laced with grooves and ridges. The colors here have been enhanced to increase surface contrasts. The violet shades extending from the top and bottom are likely due to frost particles in Ganymede’s polar regions. Future missions to Jupiter are being proposed that can search Europa and Ganymede for deep oceans that may harbor elements

Homework #2 is due Wednesday Ø Multiple choice portion: noon Ø Short answer portion: class time Homework #3 will be posted on Wednesday It will be due Tuesday, Sept. 29, 5: 00 pm Exam #1, Wednesday, Sept. 30

Radiative energy: energy carried by electromagnetic radiation (light).

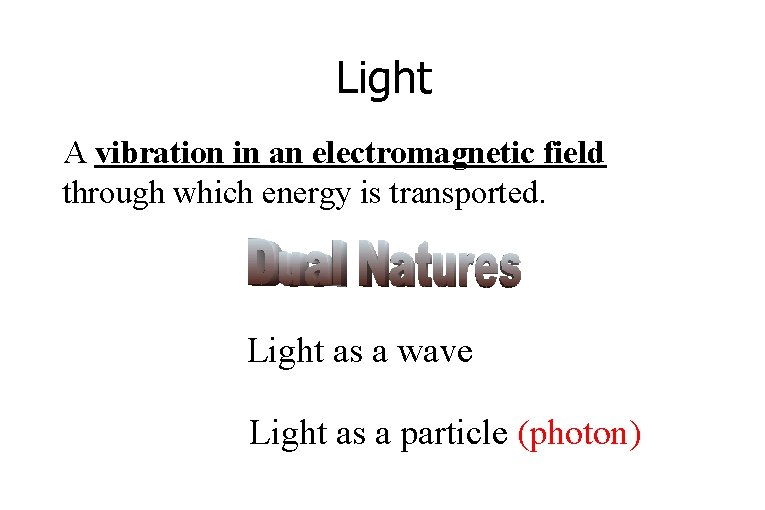
Light A vibration in an electromagnetic field through which energy is transported. Light as a wave Light as a particle (photon)

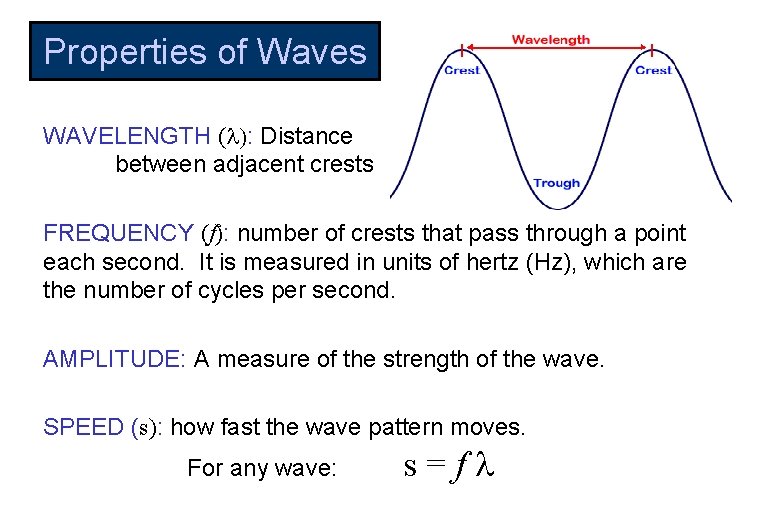
Properties of Waves WAVELENGTH ( : Distance between adjacent crests FREQUENCY (f): number of crests that pass through a point each second. It is measured in units of hertz (Hz), which are the number of cycles per second. AMPLITUDE: A measure of the strength of the wave. SPEED (s): how fast the wave pattern moves. For any wave: s=f

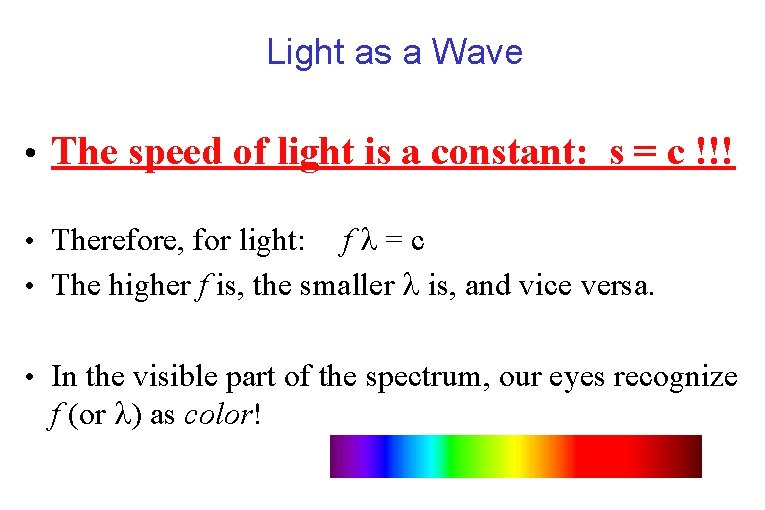
Light as a Wave • The speed of light is a constant: s = c !!! f =c • The higher f is, the smaller is, and vice versa. • Therefore, for light: • In the visible part of the spectrum, our eyes recognize f (or ) as color!

Light as a Particle v Light can also be treated as photons – packets of energy. v The energy carried by each photon depends on its frequency (color) v Energy: E = hf = hc/ [“h” is called Planck’s Constant] Shorter wavelength light carries more energy per photon.

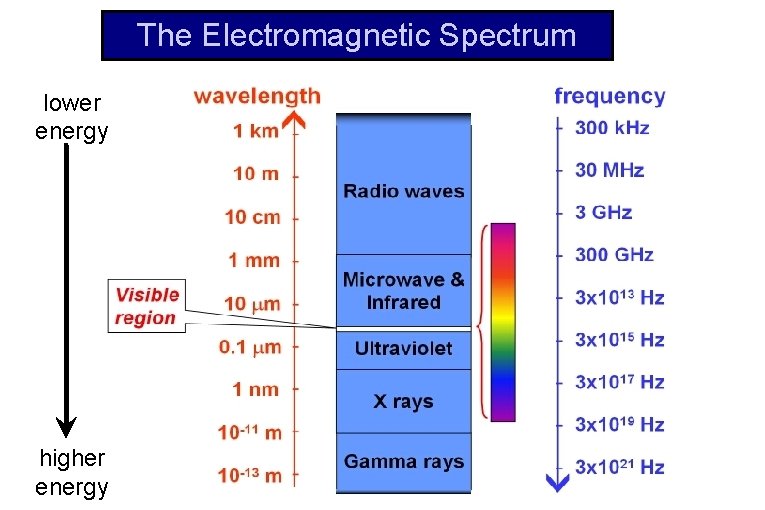
The Electromagnetic Spectrum lower energy higher energy

Light as Information Bearer Spectrum: light separated into its different wavelengths. Spectroscopy: The quantitative analysis of spectra The spectrum of an object can reveal the object’s: Composition Temperature Velocity

“Matter” and Light

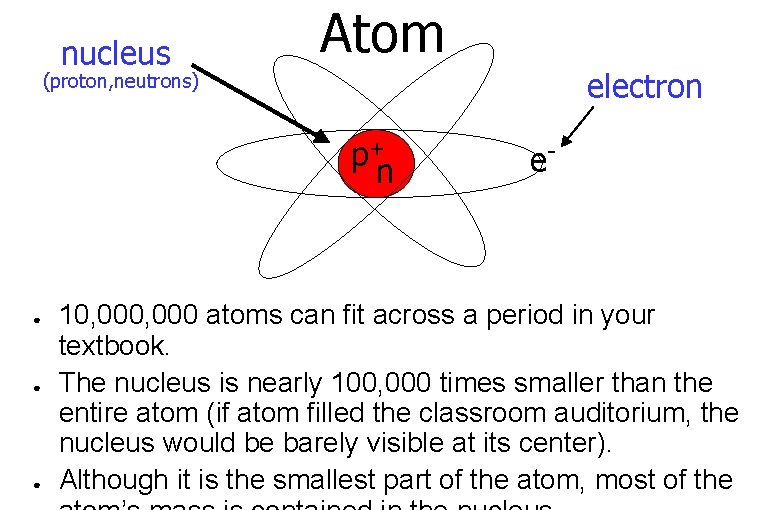
nucleus Atom electron (proton, neutrons) p+ n ● ● ● e- 10, 000 atoms can fit across a period in your textbook. The nucleus is nearly 100, 000 times smaller than the entire atom (if atom filled the classroom auditorium, the nucleus would be barely visible at its center). Although it is the smallest part of the atom, most of the

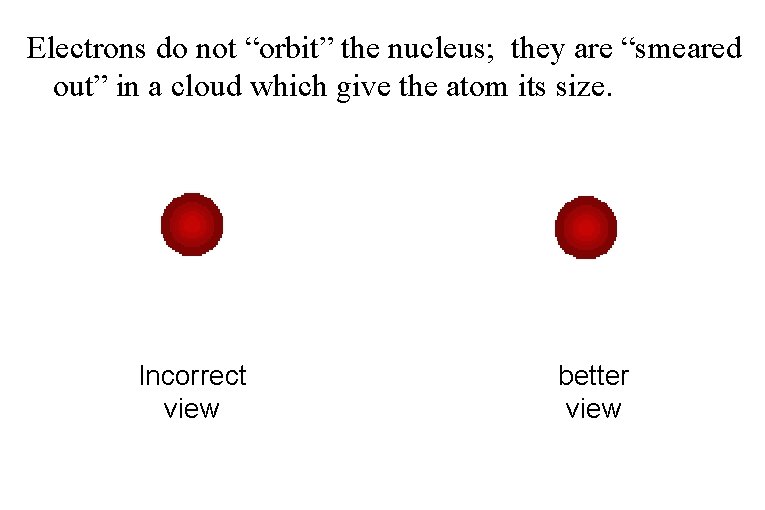
Electrons do not “orbit” the nucleus; they are “smeared out” in a cloud which give the atom its size. Incorrect view better view

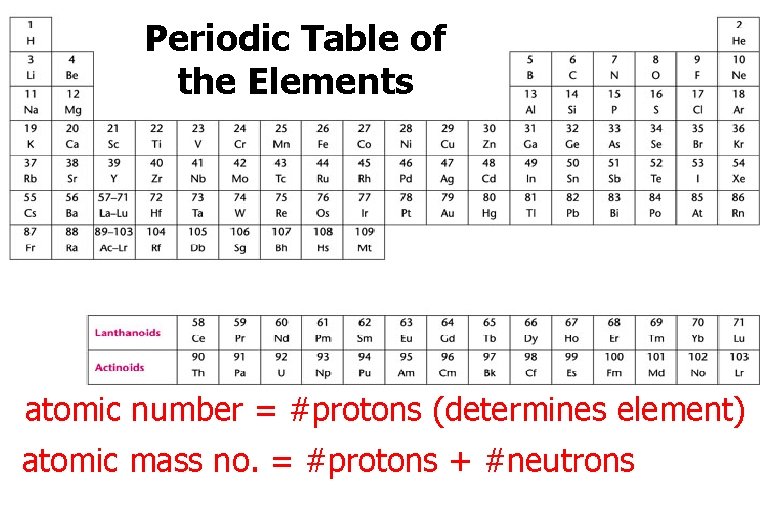
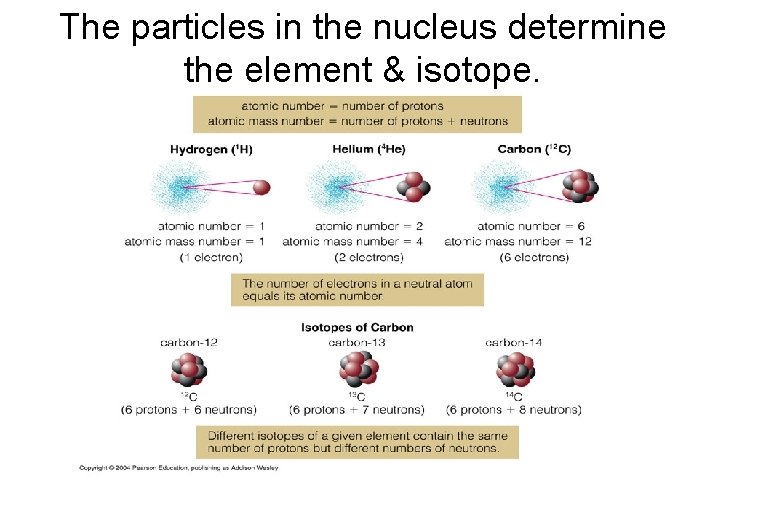
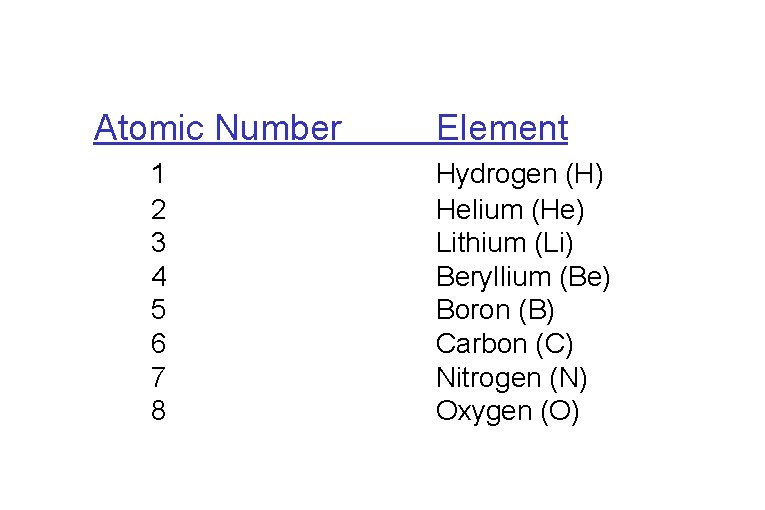
Periodic Table of the Elements atomic number = #protons (determines element) atomic mass no. = #protons + #neutrons

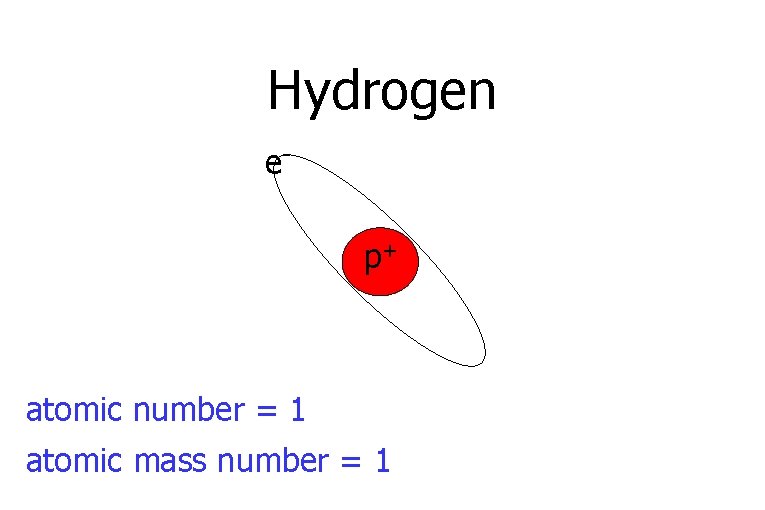
Hydrogen ep+ atomic number = 1 atomic mass number = 1

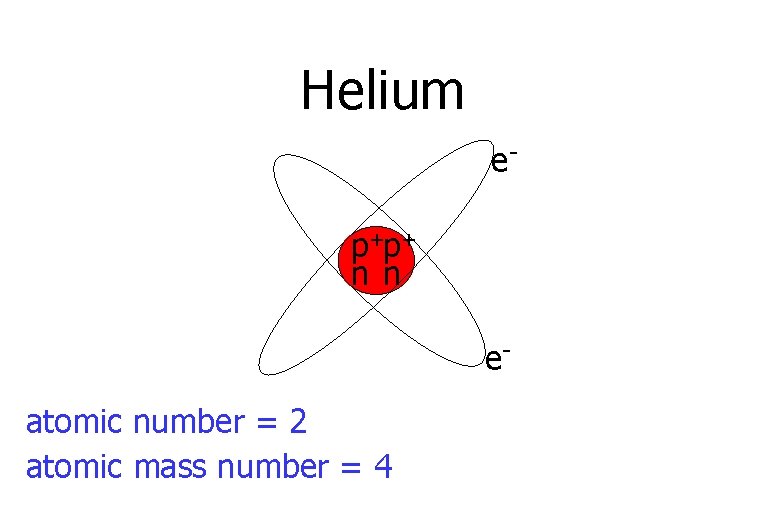
Helium ep+p+ n n eatomic number = 2 atomic mass number = 4

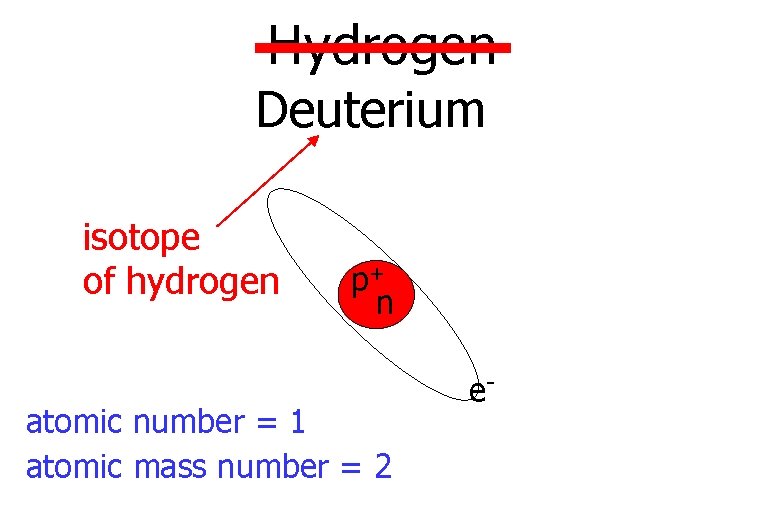
Hydrogen Deuterium isotope of hydrogen p+ n atomic number = 1 atomic mass number = 2 e-

The particles in the nucleus determine the element & isotope.

Atomic Number 1 2 3 4 5 6 7 8 Element Hydrogen (H) Helium (He) Lithium (Li) Beryllium (Be) Boron (B) Carbon (C) Nitrogen (N) Oxygen (O)

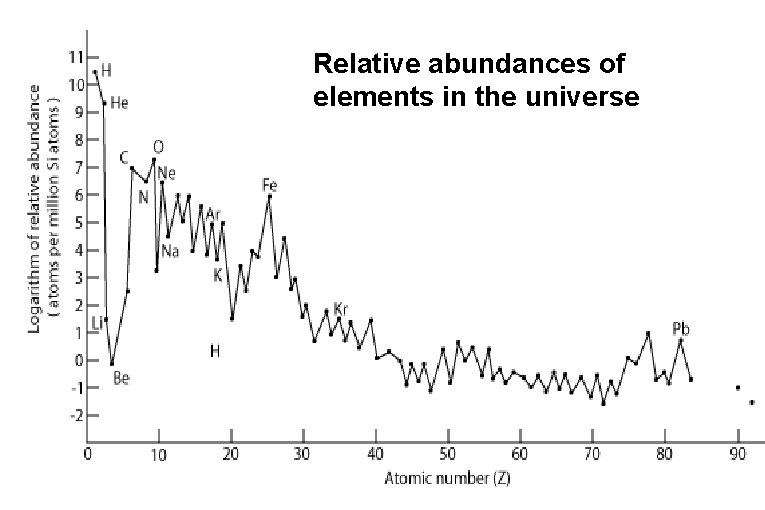
Relative abundances of elements in the universe

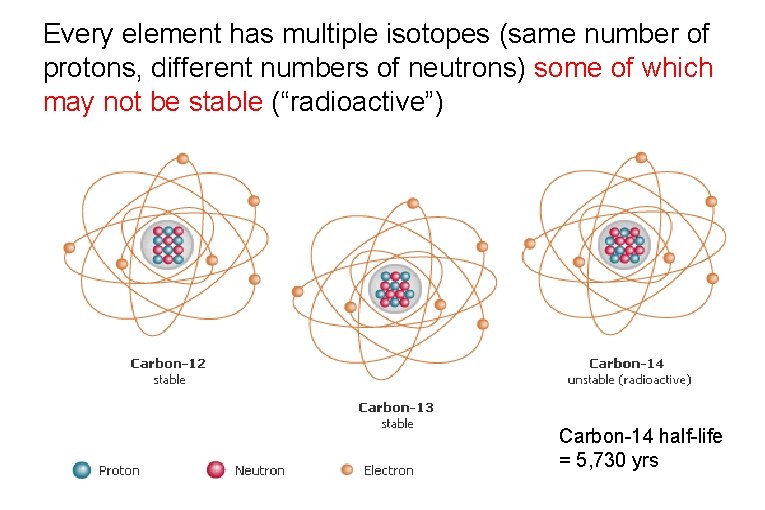
Every element has multiple isotopes (same number of protons, different numbers of neutrons) some of which may not be stable (“radioactive”) Carbon-14 half-life = 5, 730 yrs

Unstable (“radioactive”) isotopes “decay”, producing a new type of atom, i. e. , an atom of a different element, or a different isotope of the original element. One half of the atoms of an unstable isotope decay in one “half-life” of that isotope.

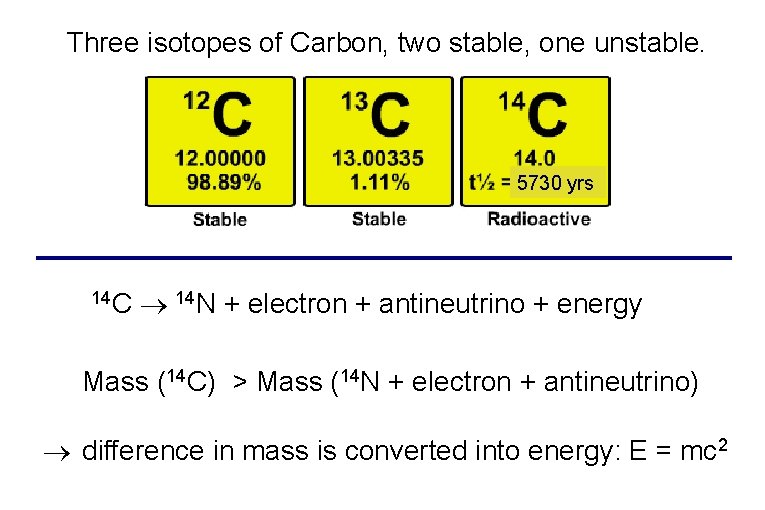
Three isotopes of Carbon, two stable, one unstable. 5730 yrs 14 C 14 N + electron + antineutrino + energy Mass (14 C) > Mass (14 N + electron + antineutrino) difference in mass is converted into energy: E = mc 2

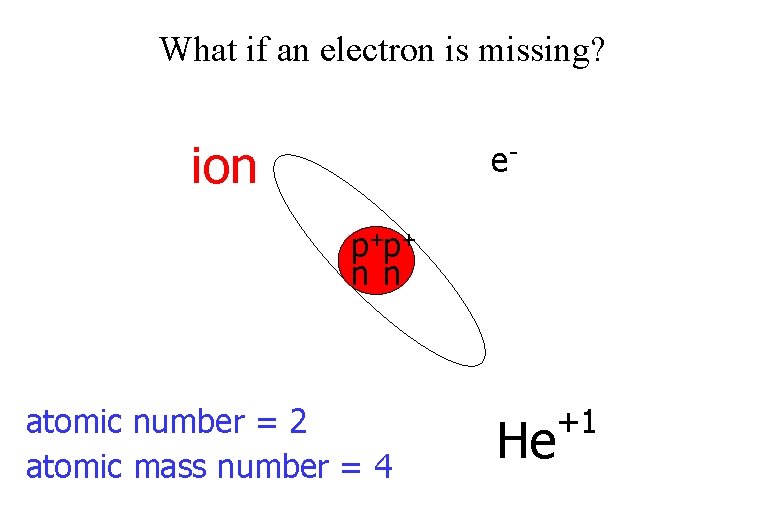
What if an electron is missing? ion ep+p+ n n atomic number = 2 atomic mass number = 4 He +1

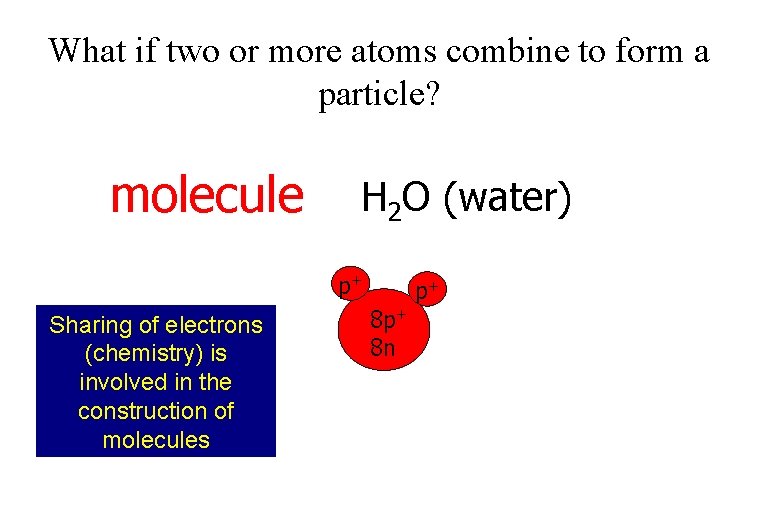
What if two or more atoms combine to form a particle? molecule H 2 O (water) p+ Sharing of electrons (chemistry) is involved in the construction of molecules 8 p+ 8 n p+

If you added a proton to an atom to create a new stable, isolated atom, you would have created… (blue) an isotope of the original element (yellow) a fission reaction (red) a different element with a positive charge (green) a neutron and a positron

If you added a proton to an atom to create a new stable, isolated atom, you would have created… (blue) an isotope of the original element (yellow) a fission reaction (red) a different element with a positive charge (green) a neutron and a positron

If you removed an electron from an atom, you would have created (blue) an isotope of the original element (yellow) a fission reaction (red) a different element with a positive charge (green) an ionized atom

If you removed an electron from an atom, you would have created (blue) an isotope of the original element (yellow) a fission reaction (red) a different element with a positive charge (green) an ionized atom

If you combined two atoms such that they shared electrons to create a new stable object, you would have created (blue) an isotope of the original element (yellow) a molecule (red) a different element (green) an ionized atom

If you combined two atoms such that they shared electrons to create a new stable object, you would have created (blue) an isotope of the original element (yellow) a molecule (red) a different element (green) an ionized atom

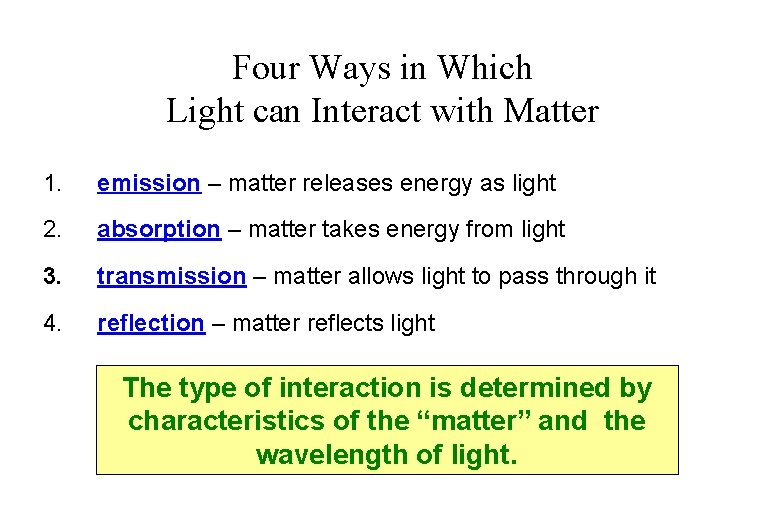
Four Ways in Which Light can Interact with Matter 1. emission – matter releases energy as light 2. absorption – matter takes energy from light 3. transmission – matter allows light to pass through it 4. reflection – matter reflects light The type of interaction is determined by characteristics of the “matter” and the wavelength of light.

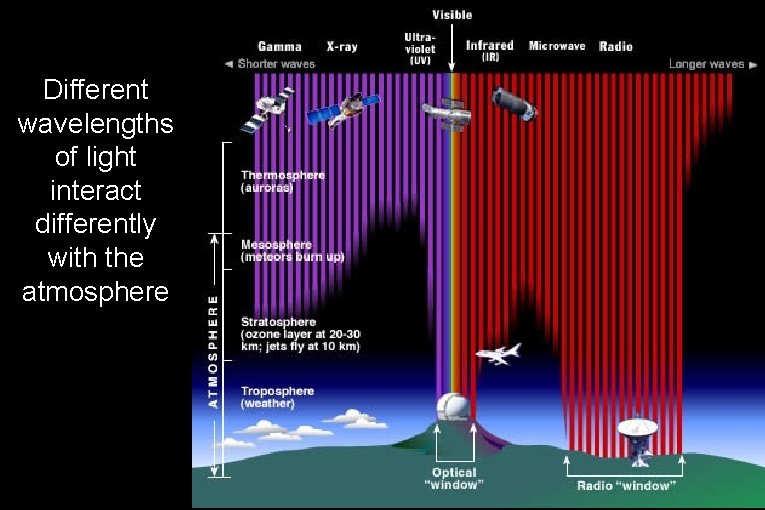
Different wavelengths of light interact differently with the atmosphere

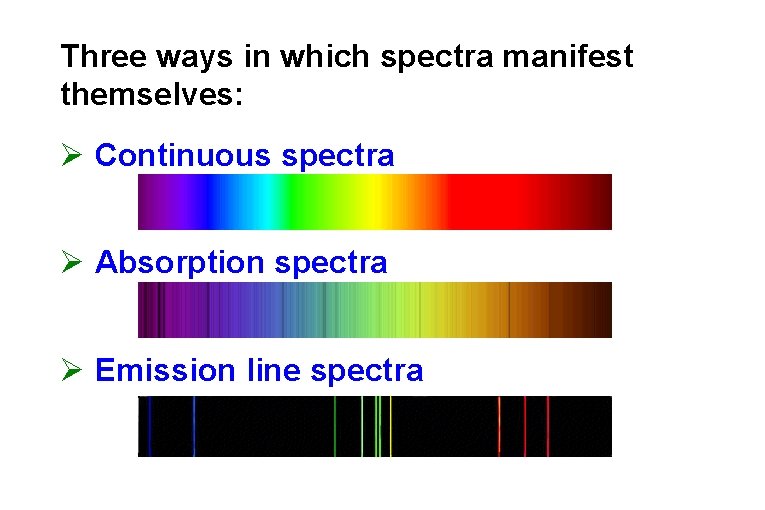
Three ways in which spectra manifest themselves: Ø Continuous spectra Ø Absorption spectra Ø Emission line spectra

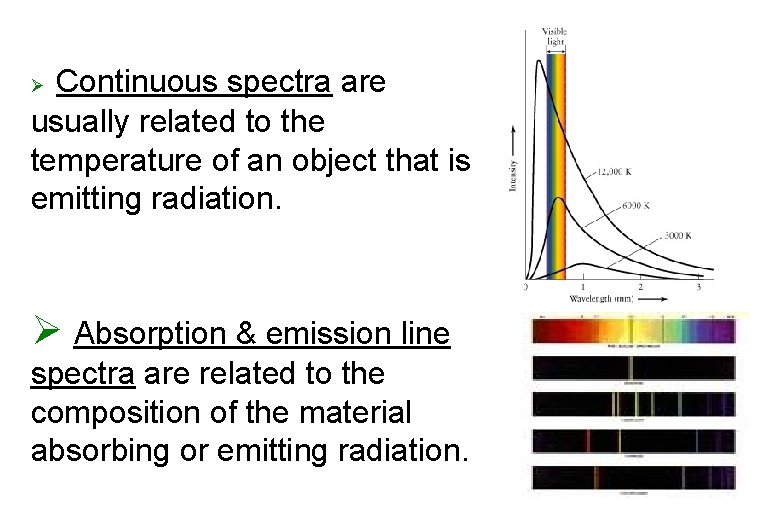
Continuous spectra are usually related to the temperature of an object that is emitting radiation. Ø Ø Absorption & emission line spectra are related to the composition of the material absorbing or emitting radiation.

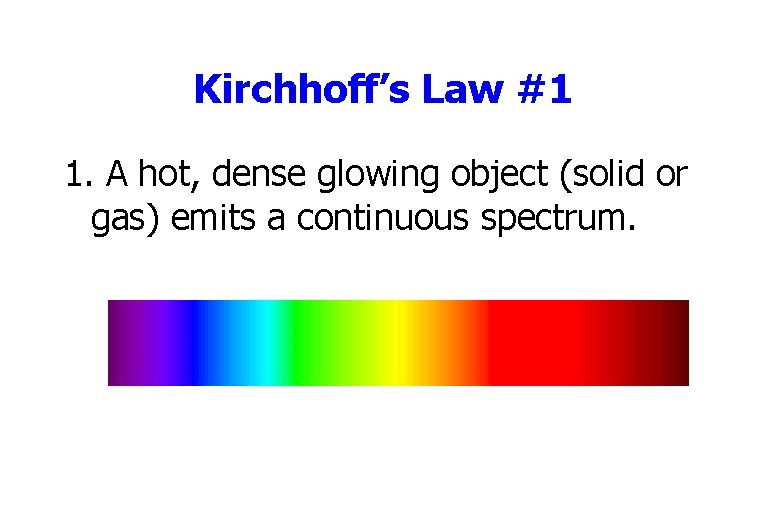
Kirchhoff’s Law #1 1. A hot, dense glowing object (solid or gas) emits a continuous spectrum.

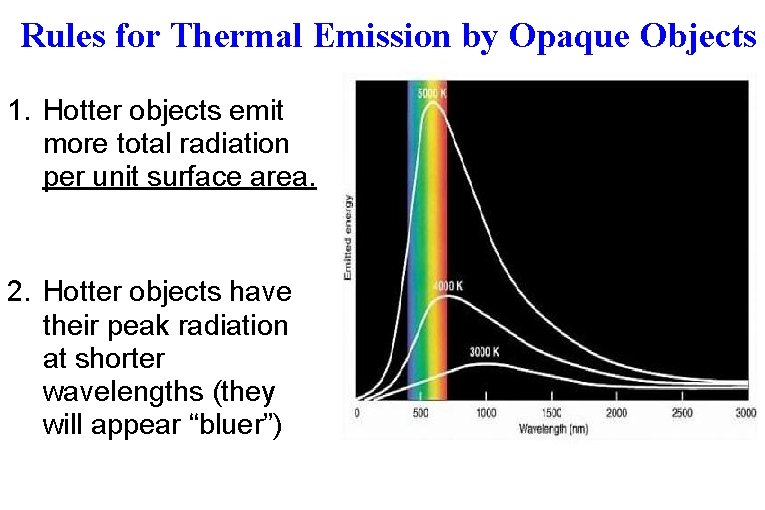
Rules for Thermal Emission by Opaque Objects 1. Hotter objects emit more total radiation per unit surface area. 2. Hotter objects have their peak radiation at shorter wavelengths (they will appear “bluer”)

The sun emits peak radiation in the yellow portion of the visible spectrum At “room temperature”, or “bodytemperature”, an object emits peak radiation in the infrared.

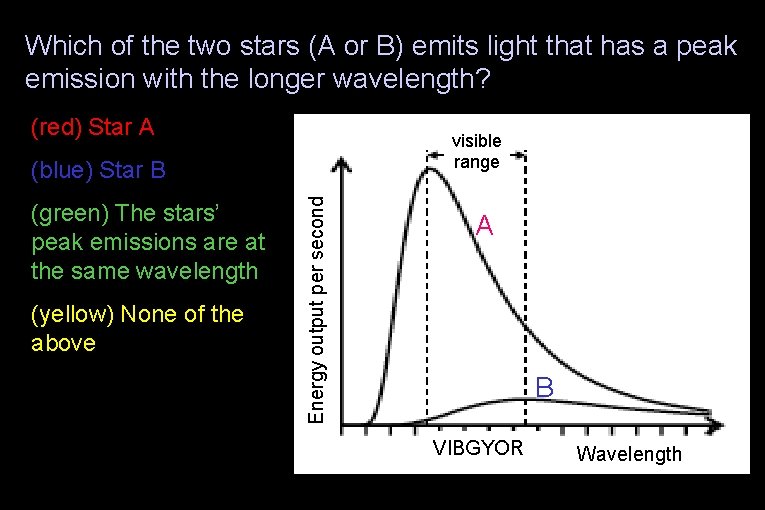
Which of the two stars (A or B) emits light that has a peak emission with the longer wavelength? (red) Star A visible range (green) The stars’ peak emissions are at the same wavelength (yellow) None of the above Energy output per second (blue) Star B A B VIBGYOR Wavelength

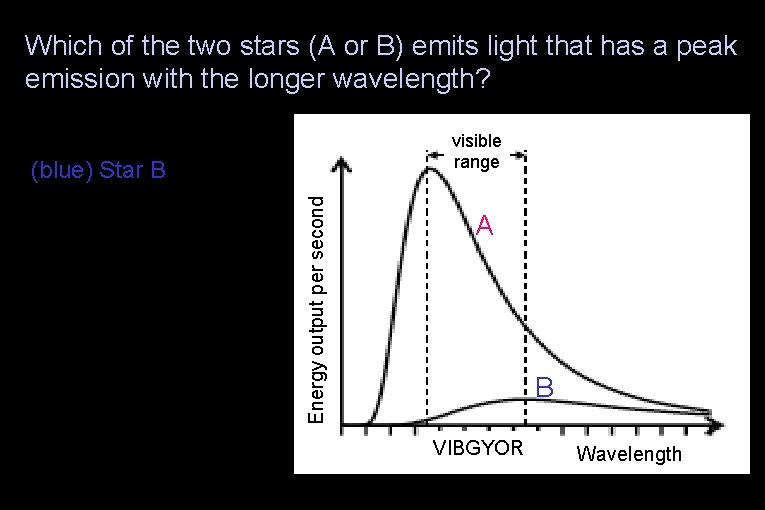
Which of the two stars (A or B) emits light that has a peak emission with the longer wavelength? (red) Star A visible range (green) The stars’ peak emissions are at the same wavelength (yellow) None of the above Energy output per second (blue) Star B A B VIBGYOR Wavelength

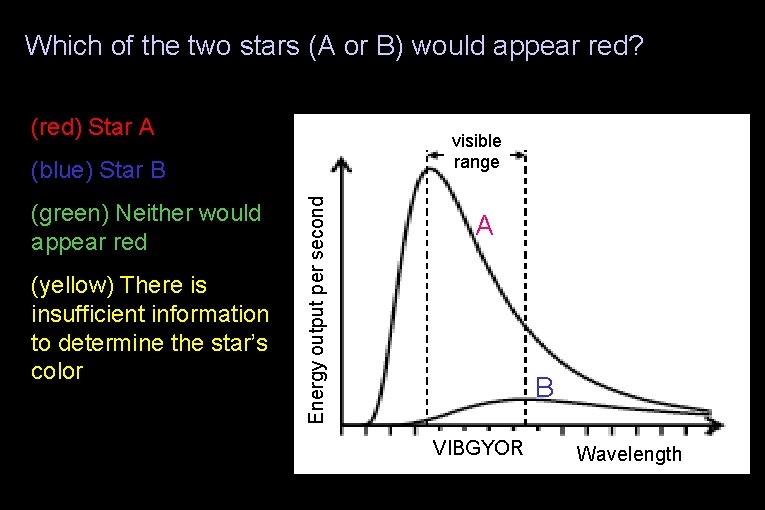
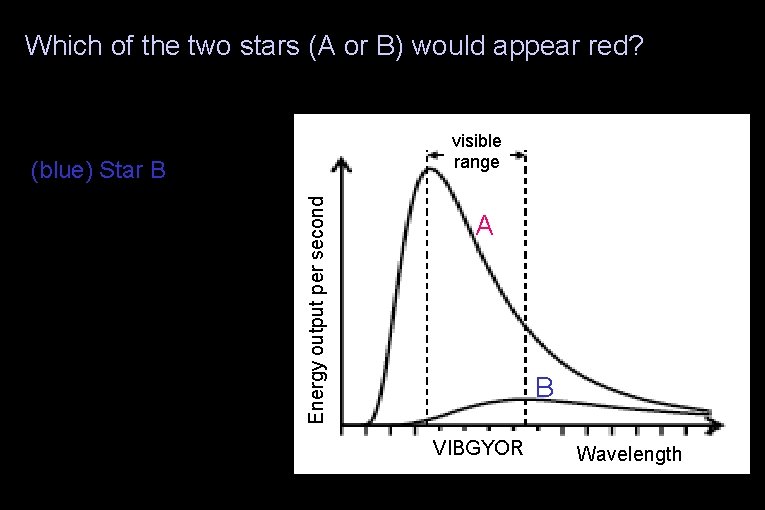
Which of the two stars (A or B) would appear red? (red) Star A visible range (green) Neither would appear red (yellow) There is insufficient information to determine the star’s color Energy output per second (blue) Star B A B VIBGYOR Wavelength

Which of the two stars (A or B) would appear red? (red) Star A visible range (green) Neither would appear red (yellow) There is insufficient information to determine the star’s color Energy output per second (blue) Star B A B VIBGYOR Wavelength

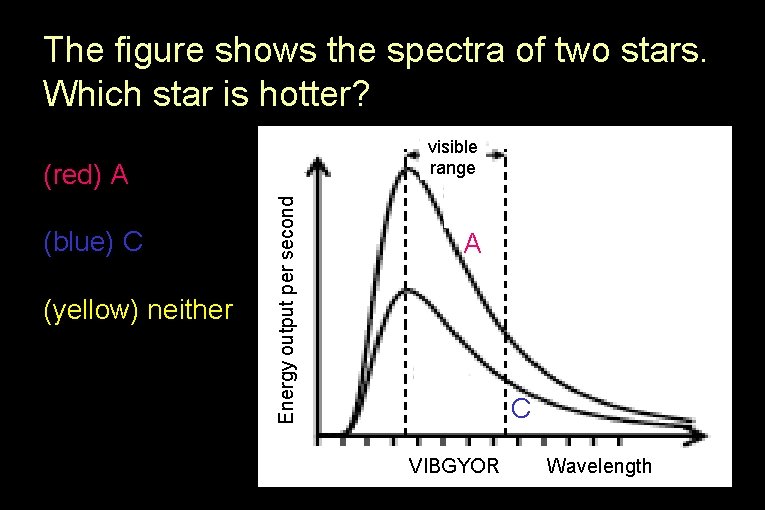
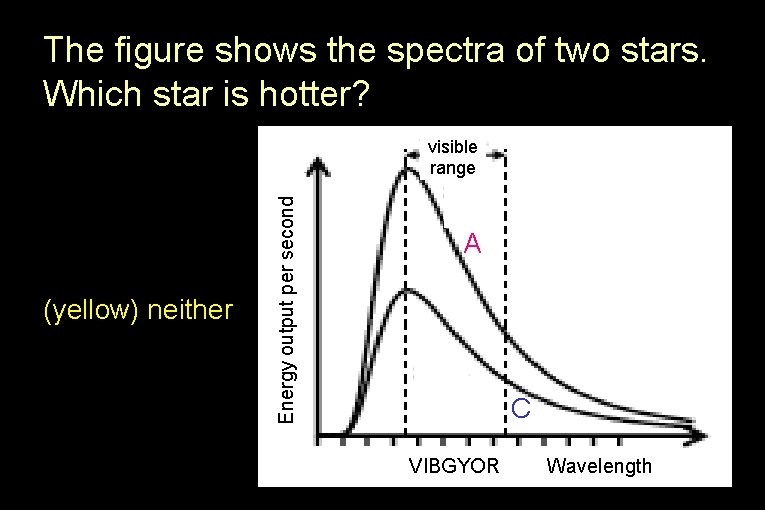
The figure shows the spectra of two stars. Which star is hotter? visible range (blue) C (yellow) neither Energy output per second (red) A A C VIBGYOR Wavelength

The figure shows the spectra of two stars. Which star is hotter? visible range (blue) C (yellow) neither Energy output per second (red) A A C VIBGYOR Wavelength

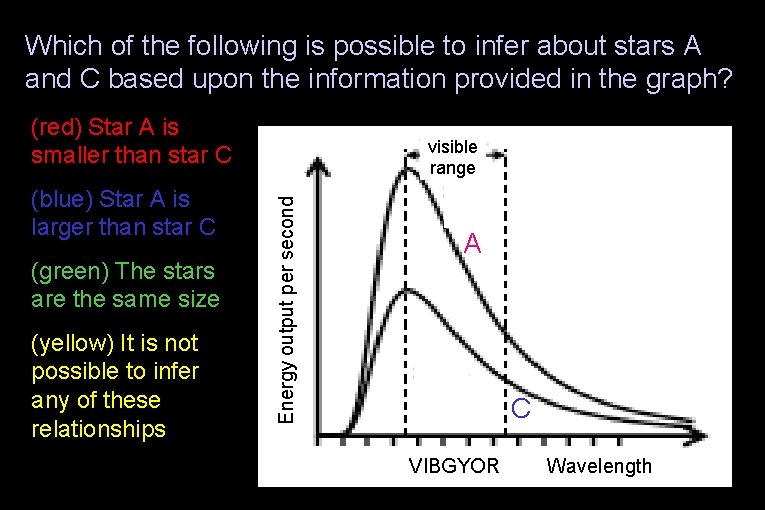
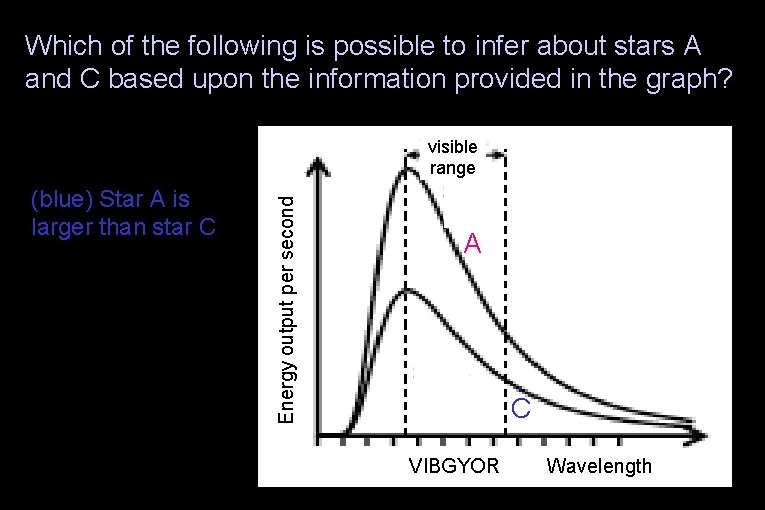
Which of the following is possible to infer about stars A and C based upon the information provided in the graph? (red) Star A is smaller than star C (green) The stars are the same size (yellow) It is not possible to infer any of these relationships Energy output per second (blue) Star A is larger than star C visible range A C VIBGYOR Wavelength

Which of the following is possible to infer about stars A and C based upon the information provided in the graph? (red) Star A is smaller than star C (green) The stars are the same size (yellow) It is not possible to infer any of these relationships Energy output per second (blue) Star A is larger than star C visible range A C VIBGYOR Wavelength
 Largest moon in the solar system
Largest moon in the solar system Eclpses
Eclpses Ganymedes
Ganymedes Cinyras
Cinyras Which moon phase occurs directly before a new moon
Which moon phase occurs directly before a new moon Which moon phase occurs directly before a new moon
Which moon phase occurs directly before a new moon Moon sister moon calendar
Moon sister moon calendar Home.hiwaay.net/ krcool/astro/moon/moon tides/
Home.hiwaay.net/ krcool/astro/moon/moon tides/ What phase is this
What phase is this Wholesale solar.com
Wholesale solar.com Solar energy is free. solar is inexhaustible
Solar energy is free. solar is inexhaustible The sun-earth-moon system worksheet answers lesson 1
The sun-earth-moon system worksheet answers lesson 1 Earth moon
Earth moon Solar system webquest answer
Solar system webquest answer Solar system sweden
Solar system sweden Uranus distance from sun
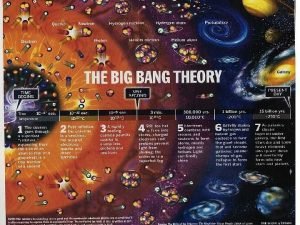
Uranus distance from sun Nebular theory comic strip
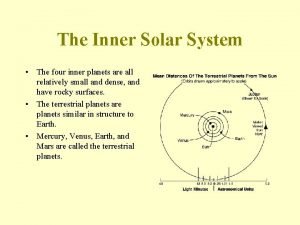
Nebular theory comic strip The four inner planets
The four inner planets Dual sun solar system
Dual sun solar system Outer solar system brainpop answers
Outer solar system brainpop answers Solar system science olympiad
Solar system science olympiad Kepler's model of the solar system
Kepler's model of the solar system Make me genius solar system
Make me genius solar system Chapter 29 our solar system
Chapter 29 our solar system Chapter 28 our solar system
Chapter 28 our solar system Chapter 23 touring our solar system
Chapter 23 touring our solar system Kepler's model of the solar system
Kepler's model of the solar system Where is the solar system located in the milky way
Where is the solar system located in the milky way Thermolite solar charging system
Thermolite solar charging system Characteristic of mars
Characteristic of mars Solar system inner and outer planets
Solar system inner and outer planets Redshift and blueshift
Redshift and blueshift Solar system jeopardy
Solar system jeopardy Solar cooling system
Solar cooling system Solar cities biogas
Solar cities biogas What separates the inner planets and outer planets
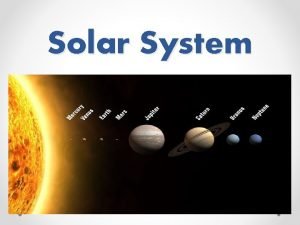
What separates the inner planets and outer planets The solar system in order
The solar system in order Solar portable alternative communications energy system
Solar portable alternative communications energy system Chapter 28 our solar system
Chapter 28 our solar system The solar system grade 8
The solar system grade 8 Www.makemegenius.com solar system
Www.makemegenius.com solar system Heraclides solar system model
Heraclides solar system model History of solar system
History of solar system Solar cloth system
Solar cloth system Natural science grade 6
Natural science grade 6 Eratosthenes solar system
Eratosthenes solar system