FUEL CELLS Chapter 7 Types of Fuel Cells







- Slides: 14

FUEL CELLS Chapter 7

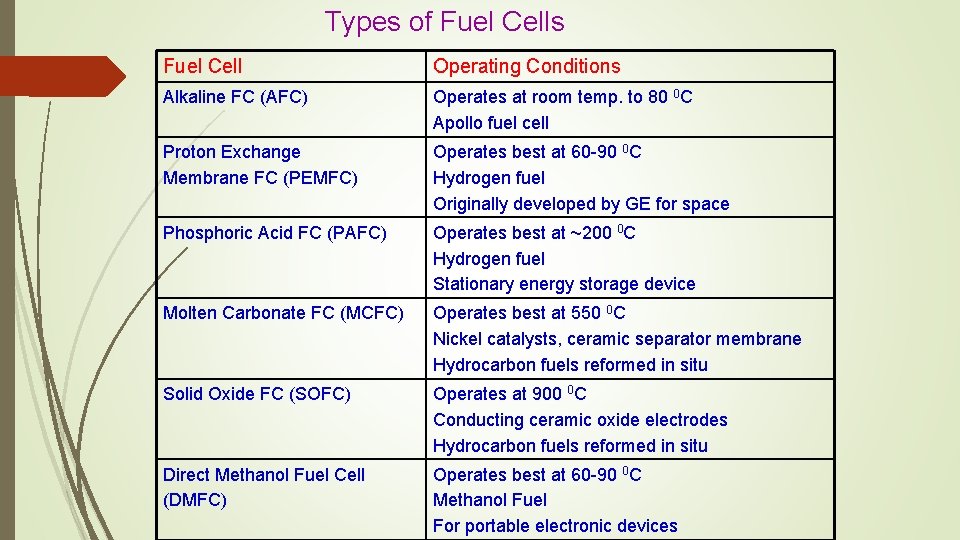
Types of Fuel Cells Fuel Cell Operating Conditions Alkaline FC (AFC) Operates at room temp. to 80 0 C Apollo fuel cell Proton Exchange Membrane FC (PEMFC) Operates best at 60 -90 0 C Hydrogen fuel Originally developed by GE for space Phosphoric Acid FC (PAFC) Operates best at ~200 0 C Hydrogen fuel Stationary energy storage device Molten Carbonate FC (MCFC) Operates best at 550 0 C Nickel catalysts, ceramic separator membrane Hydrocarbon fuels reformed in situ Solid Oxide FC (SOFC) Operates at 900 0 C Conducting ceramic oxide electrodes Hydrocarbon fuels reformed in situ Direct Methanol Fuel Cell (DMFC) Operates best at 60 -90 0 C Methanol Fuel For portable electronic devices

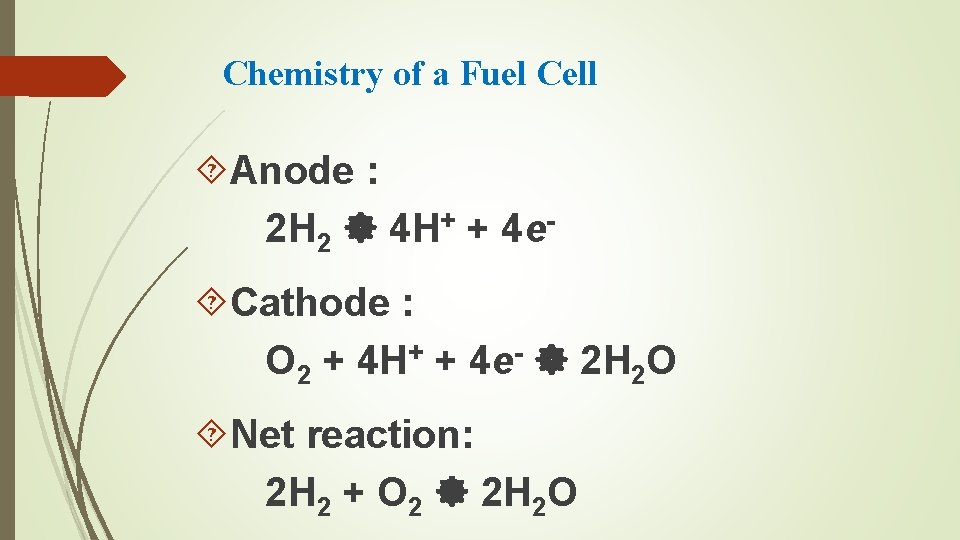
Chemistry of a Fuel Cell Anode : 2 H 2 4 H+ + 4 e Cathode : O 2 + 4 H+ + 4 e- 2 H 2 O Net reaction: 2 H 2 + O 2 2 H 2 O

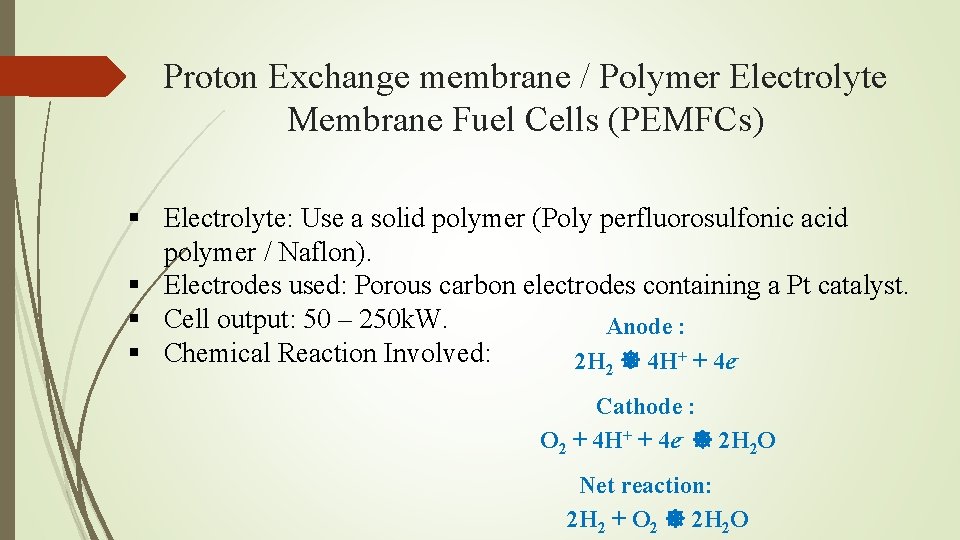
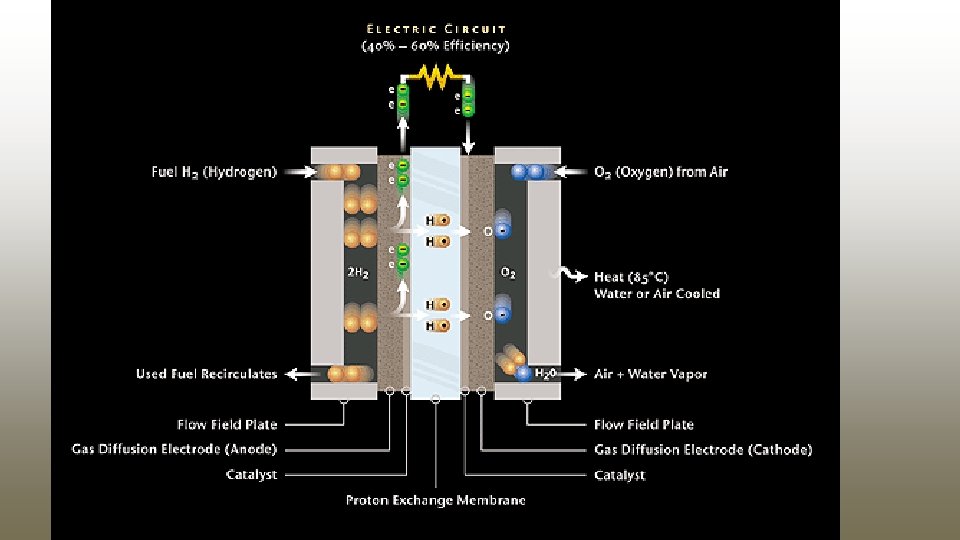
Proton Exchange membrane / Polymer Electrolyte Membrane Fuel Cells (PEMFCs)

Proton Exchange membrane / Polymer Electrolyte Membrane Fuel Cells (PEMFCs) § Electrolyte: Use a solid polymer (Poly perfluorosulfonic acid polymer / Naflon). § Electrodes used: Porous carbon electrodes containing a Pt catalyst. § Cell output: 50 – 250 k. W. Anode : § Chemical Reaction Involved: 2 H 2 4 H+ + 4 e. Cathode : O 2 + 4 H+ + 4 e- 2 H 2 O Net reaction: 2 H 2 + O 2 2 H 2 O

PEMFC


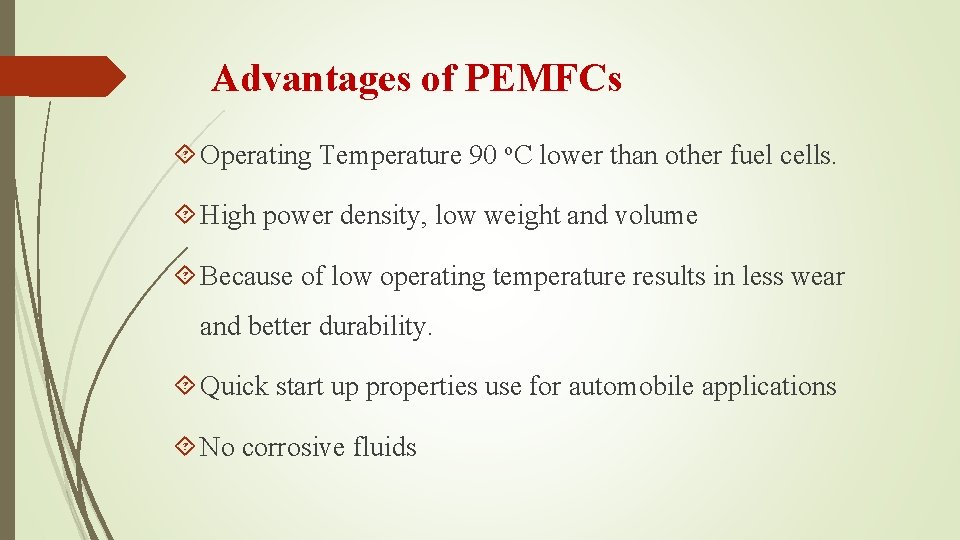
Advantages of PEMFCs Operating Temperature 90 o. C lower than other fuel cells. High power density, low weight and volume Because of low operating temperature results in less wear and better durability. Quick start up properties use for automobile applications No corrosive fluids

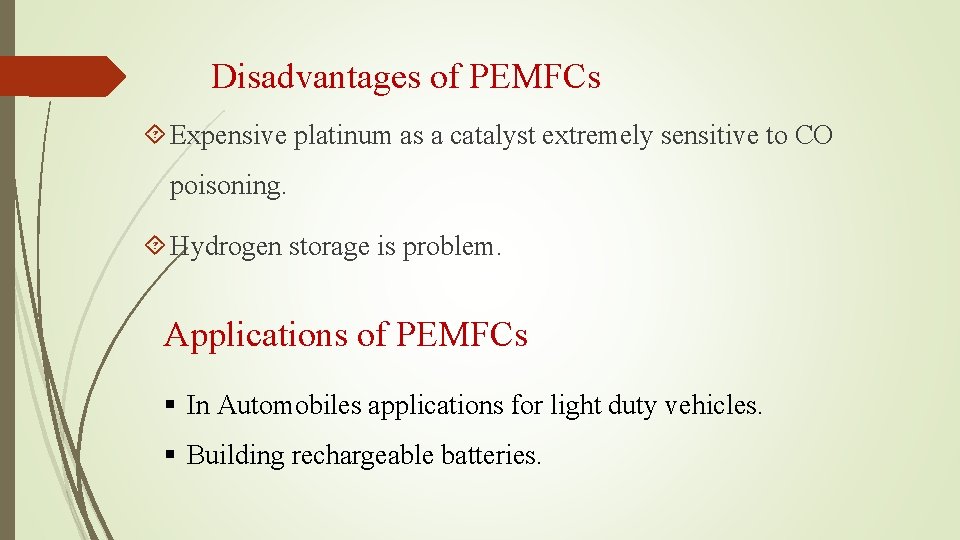
Disadvantages of PEMFCs Expensive platinum as a catalyst extremely sensitive to CO poisoning. Hydrogen storage is problem. Applications of PEMFCs § In Automobiles applications for light duty vehicles. § Building rechargeable batteries.

Phosphoric acid Fuel Cell (PAFC)

Phosphoric Acid Fuel cell World first commercially available fuel cell produced by UTC First generation of modern fuel cell. Electrolyte: Liquid Phosphoric acid retained on a silicon carbide material. Operating temperature: 150 -200 o. C. Catalyst: platinum Electrical efficiency of 40%

Advantages : Using impure hydrogen as fuel. 85% of the steam can be used for cogeneration of heat and electricity. Tolerate up to 1. 5% poisoning of CO concentration.

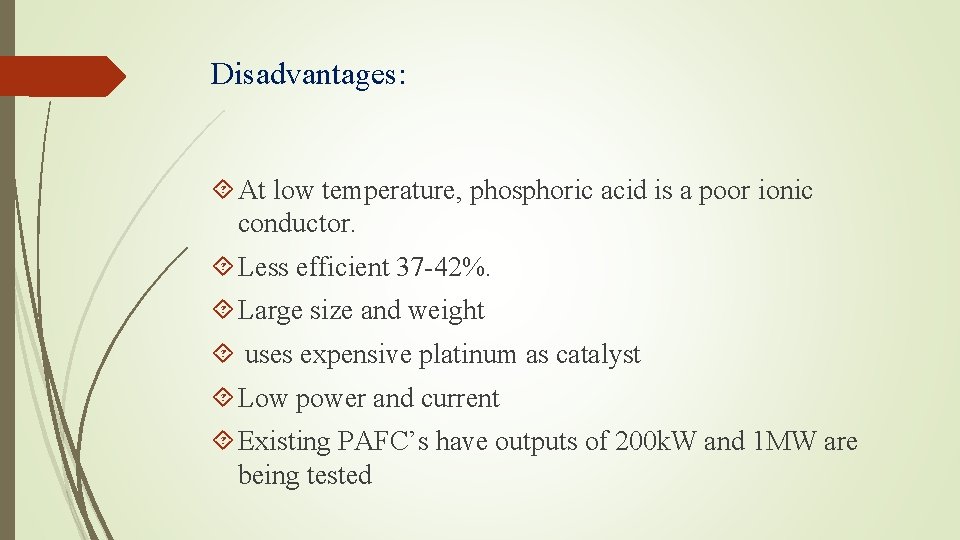
Disadvantages: At low temperature, phosphoric acid is a poor ionic conductor. Less efficient 37 -42%. Large size and weight uses expensive platinum as catalyst Low power and current Existing PAFC’s have outputs of 200 k. W and 1 MW are being tested

Applications Use for stationary power generation and in heavy and large vehicles. Used in hospitals, nursing homes, an air port terminals, waste treatment plant and for all commercial purposes.
 Chapter 8 cellular reproduction cells from cells
Chapter 8 cellular reproduction cells from cells Onodi cells and haller cells
Onodi cells and haller cells Reabsorption
Reabsorption Thyroid parafollicular cells
Thyroid parafollicular cells Haploid and diploid venn diagram
Haploid and diploid venn diagram Somatic vs germ cells
Somatic vs germ cells Red blood cells and white blood cells difference
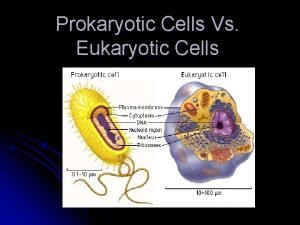
Red blood cells and white blood cells difference Eukaryotic vs prokaryotic cell
Eukaryotic vs prokaryotic cell Animal cells and plant cells venn diagram
Animal cells and plant cells venn diagram Prokaryotic cells
Prokaryotic cells Cell organelle jeopardy
Cell organelle jeopardy Masses of cells form and steal nutrients from healthy cells
Masses of cells form and steal nutrients from healthy cells Label
Label What cell type
What cell type Prokaryotic cells vs eukaryotic cells
Prokaryotic cells vs eukaryotic cells