FSIS Directive 10 800 1 Revision 1 District














- Slides: 16

FSIS Directive 10, 800. 1 Revision 1 District Correlation March 13, 2014 CDR Catherine Rockwell, DVM OPPD RIMS

FSIS Directive 10, 800. 1 Revision 1 Issued March 3, 2014 n Revised to replace previous version issued in July 2007. n

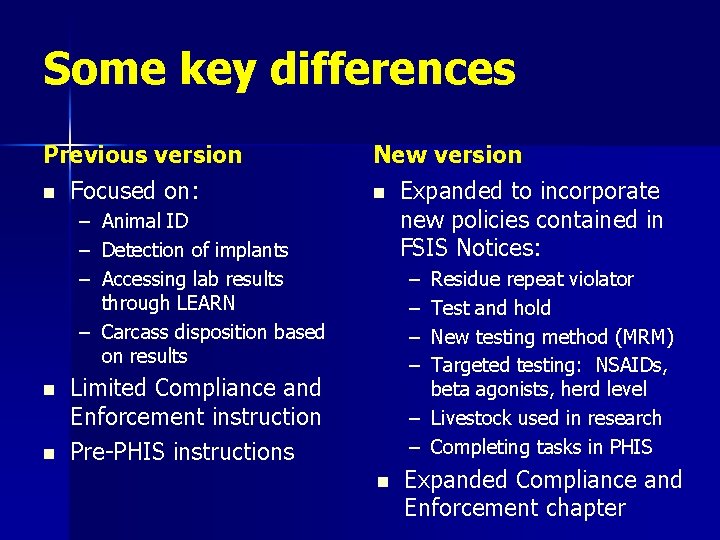
Some key differences Previous version n Focused on: – Animal ID – Detection of implants – Accessing lab results through LEARN – Carcass disposition based on results n n New version n Expanded to incorporate new policies contained in FSIS Notices: – – Residue repeat violator Test and hold New testing method (MRM) Targeted testing: NSAIDs, beta agonists, herd level – Livestock used in research – Completing tasks in PHIS Limited Compliance and Enforcement instruction Pre-PHIS instructions n Expanded Compliance and Enforcement chapter

Revised version n n Hyperlinked Table of Contents Document flow is more closely aligned with IPP workflow Program Areas of Responsibilities section included as an attachment Links to resources to aid IPP in completing residue verification tasks

Revised version n Expanded to include step-by-step instructions for completing tasks in PHIS § Directed residue testing § KIS™ and other inspector-generated testing

Revised version n Incorporates instructions from FSIS Notices and instructions on pathologies and conditions warranting in-plant testing previously contained in FSIS Directive 10, 220. 3 ü FAST testing discontinued ü Animals exhibiting any clinical or post mortem signs of septicemia or toxemia are to be tested for residue.

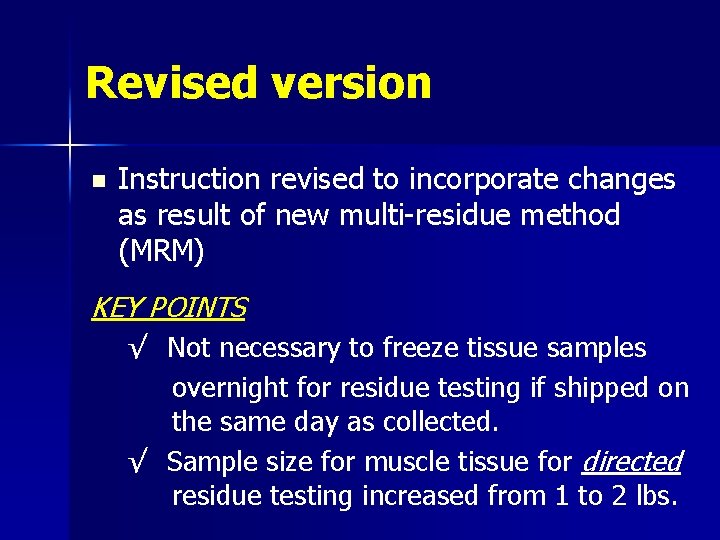
Revised version n Instruction revised to incorporate changes as result of new multi-residue method (MRM) KEY POINTS √ Not necessary to freeze tissue samples overnight for residue testing if shipped on the same day as collected. √ Sample size for muscle tissue for directed residue testing increased from 1 to 2 lbs.

Revised version n Incorporates instructions to IPP on verifying an establishment holds or controls the livestock carcass and its parts that is subject to directed residue testing pending results. ü Poultry carcasses not required to be held but it is recommended ü Carcasses subject to inspector-generated residue testing are retained by FSIS pending test results.

FSIS Directive 10, 800. 1, Rev. 1 Walk-through General Information I. • • Purpose Cancellations Key points Background Sampling Projects Under NRP II. • • Directed Inspector generated Imports National Security

FSIS Directive 10, 800. 1, Rev. 1 Walk-through Circumstances Warranting Inspector Generated Sampling III. • • Pathologies and Conditions Increased sampling frequency KIS™ testing of bob veal NSAID testing Beta agonist testing Testing of Show animals Testing at a herd level Livestock Used in Research

FSIS Directive 10, 800. 1, Rev. 1 Walk-through IPP Responsibilities for Sample Collection IV. • • Ordering sampling supplies Collecting Animal Identification and Supplier Info Conducting KIS™ testing Conducting Inspector-generated other than KIS™ Conducting Directed sampling tasks Sample packaging and shipping Accessing lab test results IPP actions upon reporting of test results

FSIS Directive 10, 800. 1, Rev. 1 Walk-through V. Compliance and Enforcement • IPP responsibilities • • Verify reassessment Veal calves with implants Test and hold Actions following FSIS violative residue result • District Office responsibilities • Documenting verification results in PHIS

FSIS Directive 10, 800. 1, Rev. 1 Walk-through VI. Data Analysis and Questions • Data analysis and trends reporting • Submitting questions through ask. FSIS

FSIS Directive 10, 800. 1, Rev. 1 Walk-through Attachments: 1. 2. 3. Program Area Responsibilities Completing Residue Sampling Tasks in PHIS Flow Chart: Regulatory Actions – Repeat Violators

FSIS Directive 10, 800. 1, Rev. 1 Walk-through Related Documents 1. 2. Animal ID: Examples of Official Ear Tags KIS™ Test Sampling Instruction Booklet Related Links: 1. 2. Recording In-plant Residue Test results Scheduling and Submitted Directed Lab Samples

Questions?
 Fsis overtime directive
Fsis overtime directive Label submission and approval system (lsas)
Label submission and approval system (lsas) What are siluriformes
What are siluriformes Lsas usda
Lsas usda Fsis notice 34 21
Fsis notice 34 21 Ask fsis
Ask fsis Fsis directives
Fsis directives 200+400+600+800
200+400+600+800 Passive voice revision
Passive voice revision Army directive 2016-34
Army directive 2016-34 Packaging and packaging waste directive
Packaging and packaging waste directive What is directive function
What is directive function Extern assembler directive
Extern assembler directive Unfair 2006
Unfair 2006 Advanced directive
Advanced directive Quality roles and responsibilities
Quality roles and responsibilities Directive question examples
Directive question examples




























