Formal Charge Formal charge is helpful in determining













- Slides: 23

Formal Charge • Formal charge is helpful in determining which structure in a resonance group is actually the one with lowest energy • Formal charge takes into account the electrons an atom actually “owns” as well as the electrons shared by covalent bonding – Remember that when we draw Lewis Structures, we just throw the valence electrons into a pile and then distribute them around the molecule to satisfy the octet rule. – Formal Charge sets the record straight, so to speak.

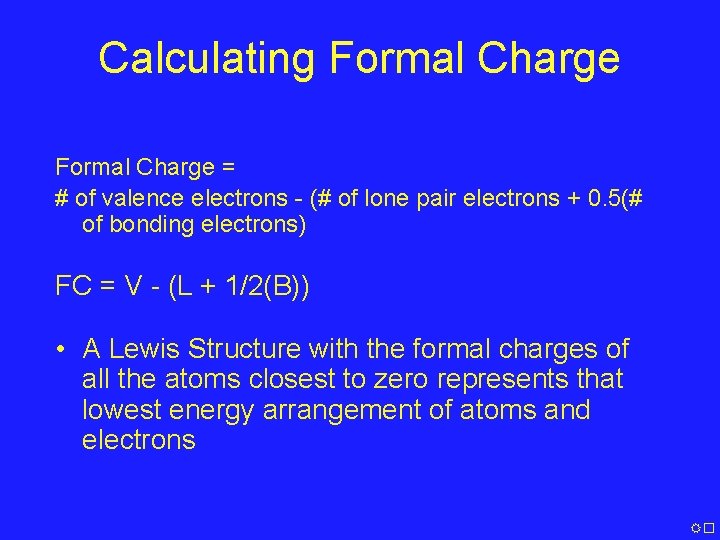
Calculating Formal Charge = # of valence electrons - (# of lone pair electrons + 0. 5(# of bonding electrons) FC = V - (L + 1/2(B)) • A Lewis Structure with the formal charges of all the atoms closest to zero represents that lowest energy arrangement of atoms and electrons �

Exceptions to the Octet Rule • Some atoms just don’t follow the octet rule and can form compounds that give odd numbers of electrons or more than 8 electrons in the valence shell 1. Radicals 2. Phosphorous, sulfur, chlorine and Group 13/III (Boron and Aluminum)

Radicals • Radicals are VERY highly reactive compounds in which a single unpaired electron exists • Biradicals are molecules with two unpaired electrons • Radicals are responsible for the effects of aging in living cells – The compounds may form from metabolic reactions (Redox reactions in the electron transfer chain, for example) or from chemical exposure – Antioxidants are compounds that can quickly and safely react with radicals before they can damage tissues

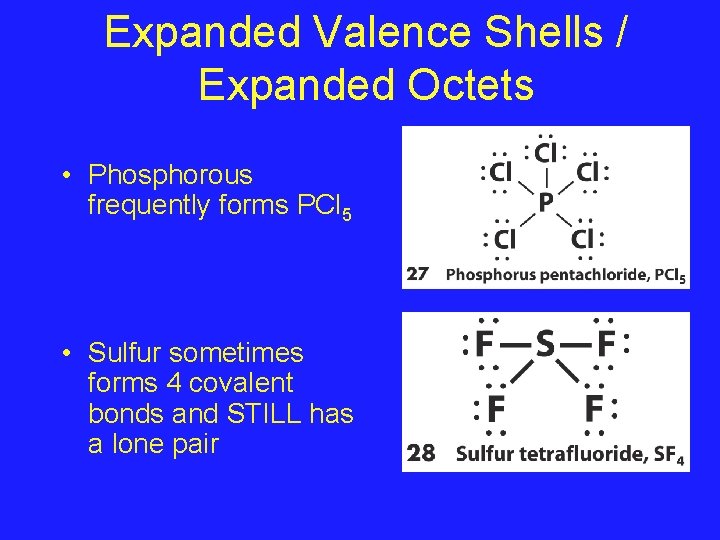
Expanded Valence Shells / Expanded Octets • Central atoms that have empty dorbitals sometimes form compounds that give them 10 or more valence electrons • Only non-metals in periods 3 or later may expand their valence shells

Expanded Valence Shells / Expanded Octets • Phosphorous frequently forms PCl 5 • Sulfur sometimes forms 4 covalent bonds and STILL has a lone pair

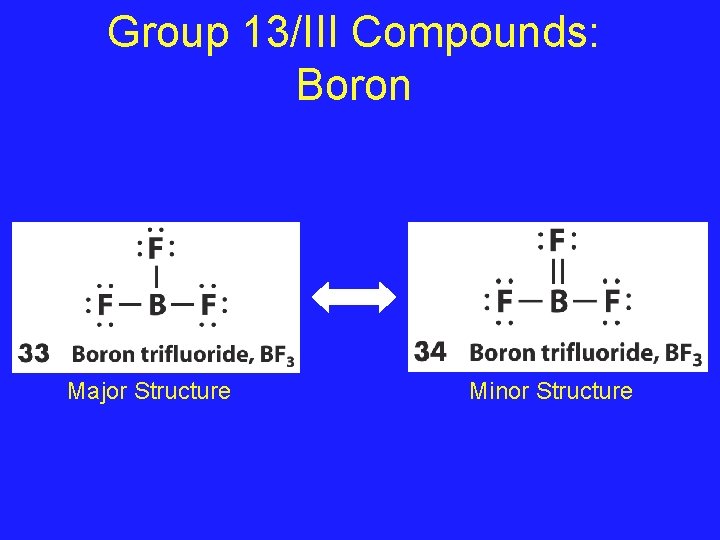
Group 13/III Compounds • Boron and Aluminum (Problem Children!) • Boron is perfectly happy with only 6 valence electrons • It forms BF 3 and instead of forming a double bond with one of the Fluorines, the major structure is one with 3 single bonds

Group 13/III Compounds: Boron Major Structure Minor Structure

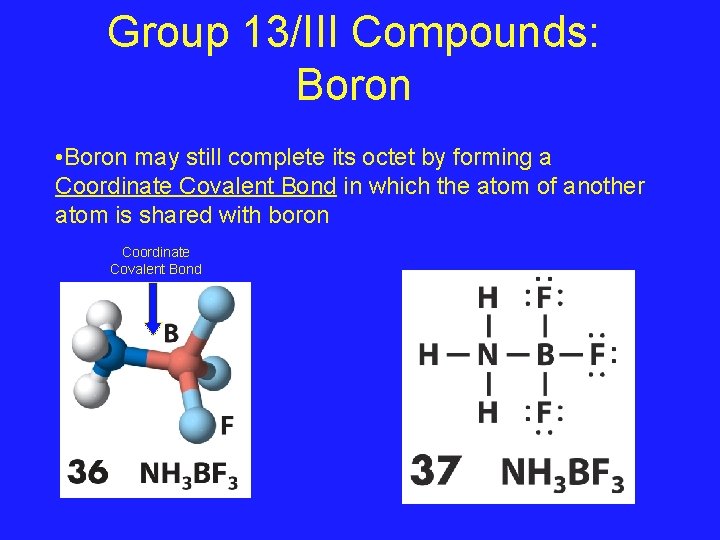
Group 13/III Compounds: Boron • Boron may still complete its octet by forming a Coordinate Covalent Bond in which the atom of another atom is shared with boron Coordinate Covalent Bond

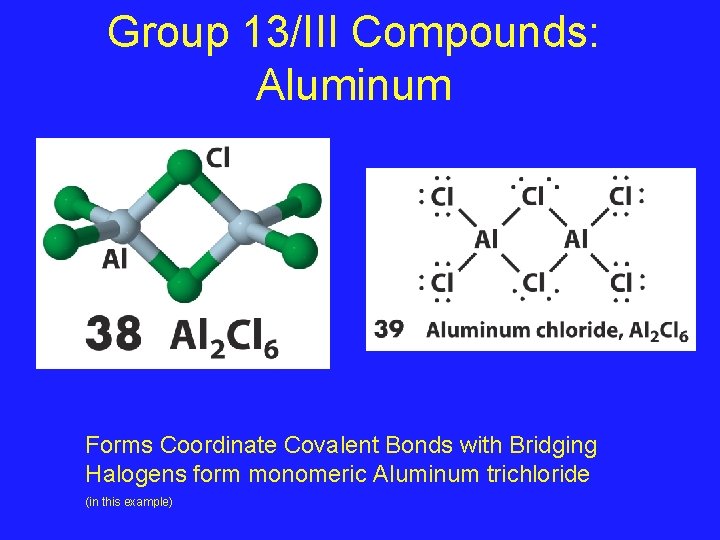
Group 13/III Compounds: Aluminum Forms Coordinate Covalent Bonds with Bridging Halogens form monomeric Aluminum trichloride (in this example)


Keeping It Real: Electronegativity • To some extent, we are still working out the details and models of how exactly bonding works • We could look at every bond as a resonance hybrids of covalent AND ionic bonds �

Electronegativity • If we look at Hydrogen chloride though, we see a different story… – The 2 Ionic resonance structures have VERY different energies • We kinow from the electron affinity of chlorine that it very much wants the electron – In fact, it doesn’t share the electron in a covalent bond very well at all • HCl is an example of a Polar Covalent Bond – The ionic contribution of one of the possible resonance structures is greater than the other(s)

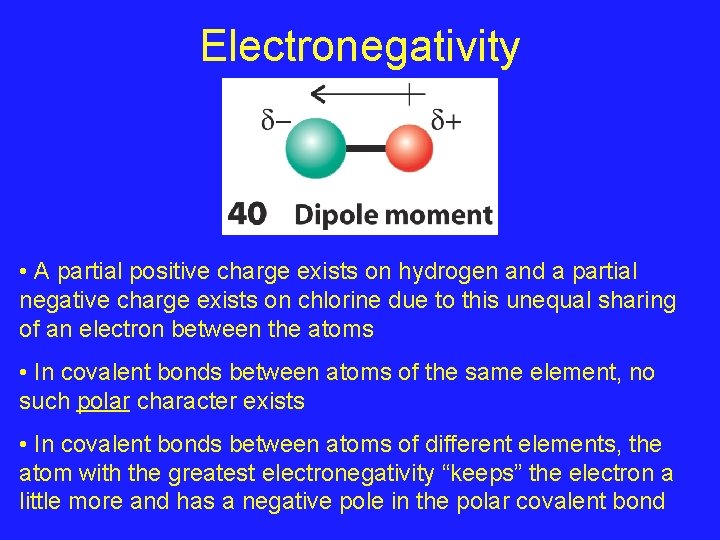
Electronegativity • A partial positive charge exists on hydrogen and a partial negative charge exists on chlorine due to this unequal sharing of an electron between the atoms • In covalent bonds between atoms of the same element, no such polar character exists • In covalent bonds between atoms of different elements, the atom with the greatest electronegativity “keeps” the electron a little more and has a negative pole in the polar covalent bond

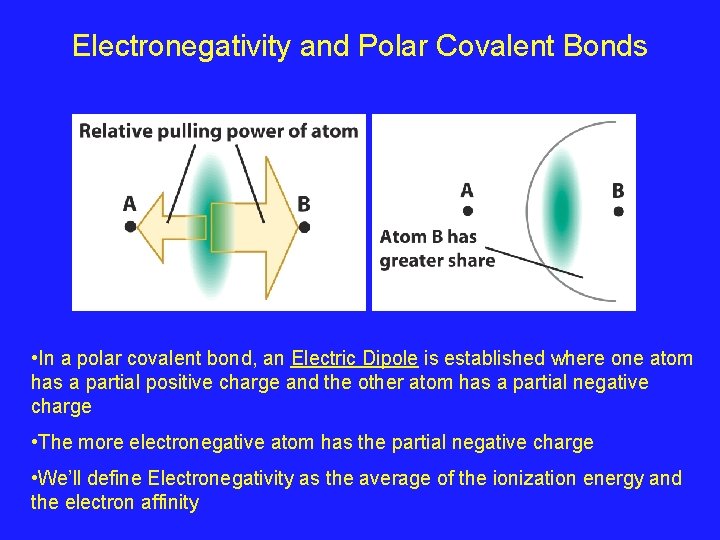
Electronegativity and Polar Covalent Bonds • In a polar covalent bond, an Electric Dipole is established where one atom has a partial positive charge and the other atom has a partial negative charge • The more electronegative atom has the partial negative charge • We’ll define Electronegativity as the average of the ionization energy and the electron affinity

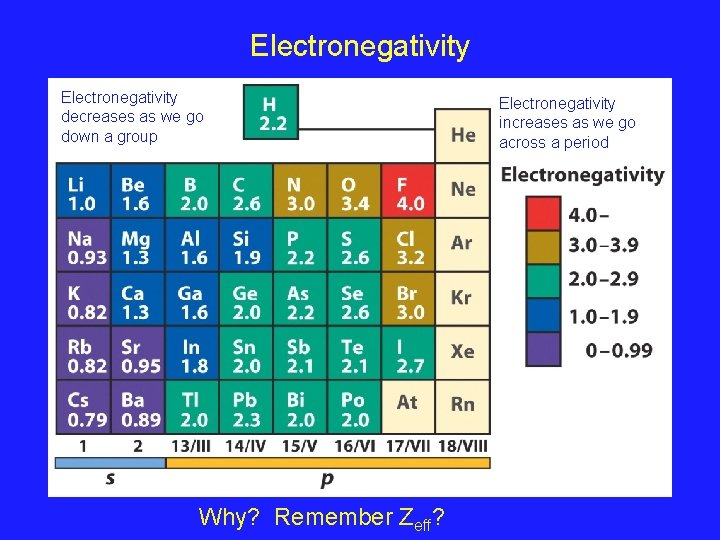
Electronegativity decreases as we go down a group Why? Remember Zeff? Electronegativity increases as we go across a period

Polarizability and Ionic Bonds • In an ionic bond, a cation and an anion are associated by their Coulombic attraction • The positive electron “pulls” at the electron cloud of the anion and gives the ionic bond more covalent tendencies • Atoms and ions that undergo large distortions of the electron cloud are said to be Highly Polarizable – The larger the anion, the more polarizable it is due to the distance of the electrons from the nucleus

Polarizability and Ionic Bonds • Atoms or ions that can cause large distortions have a high Polarizing Power – Small, highly charged cations like Al 3+ have high polarizing power – The small cation can get closer to the anion and can exert its force on the anions electron cloud • Cationic polarizing power increases from left to right across a period and decreases going down a group • The more polarized the bond is, the more covalent-like the bond becomes

Bond Strengths and Lengths • Bond strength is measured by the energy necessary/required to break it • Bond strength is proportional to the distance between the atoms in a bond – The closer two atoms are to each other, the more energy it takes to break their bond • Multiple bonds require more energy to break than single bonds

Factors Affecting Bond Strength 1. Multiple Bonds 1. Multiple bonds are not as strong as two or three times the single bond dissociation energy due to the repulsion between electrons in the multiple bond 2. Resonance 1. The delocalized electrons cause single bonds to take on some multiple bond characteristics (ie: They get shorter and the dissociation energy is higher than would be expected) 3. Lone Pairs 1. Lone pairs cause repulsion of other lone pairs or bonds and weaken neighboring covalent bonds 1. Can also affect geometry as we’ll see next chapter

Bond Strength: Summary 1) Multiple bonds increase bond strength 2) Lone pairs decrease bond strength of neighboring bonds 3) Bond strength decreases as Atomic radius increases 4) Resonance strengthens bonds

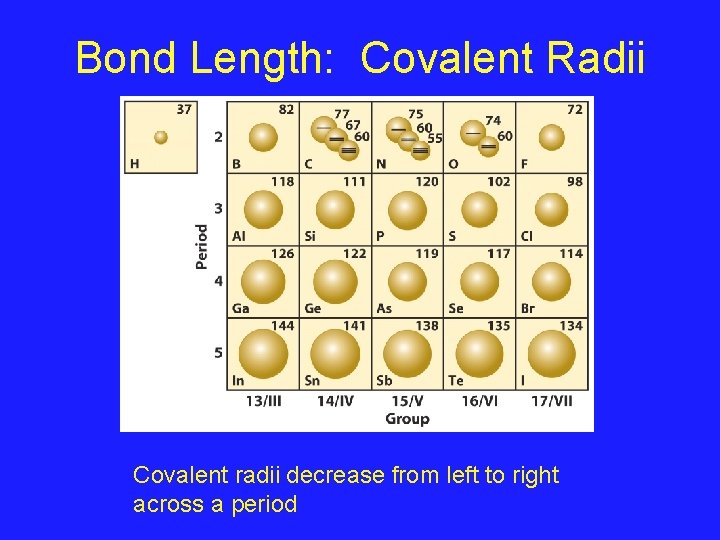
Bond Length • Bond length is defined as the distance between the centers of 2 atoms joined by a covalent bond • The stronger the bond, the shorter the bond length – Multiple bonds are shorter than single bonds • Each atom in a bond makes a contribution to the bond length called the Covalent Radius – Bond length is the sum of the covalent radii of the 2 atoms in a bond

Bond Length: Covalent Radii Covalent radii decrease from left to right across a period