Fission Fusion Why so much energy Fission Reactions

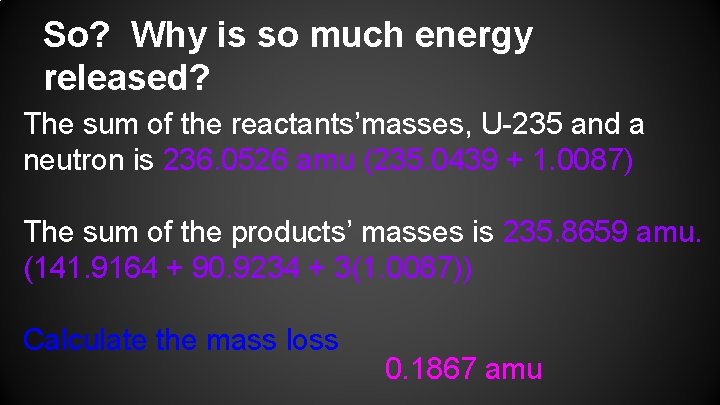






- Slides: 15

Fission & Fusion Why so much energy? ? ?

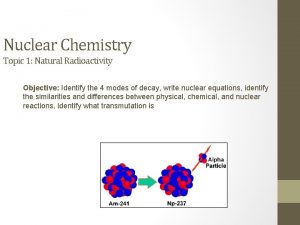
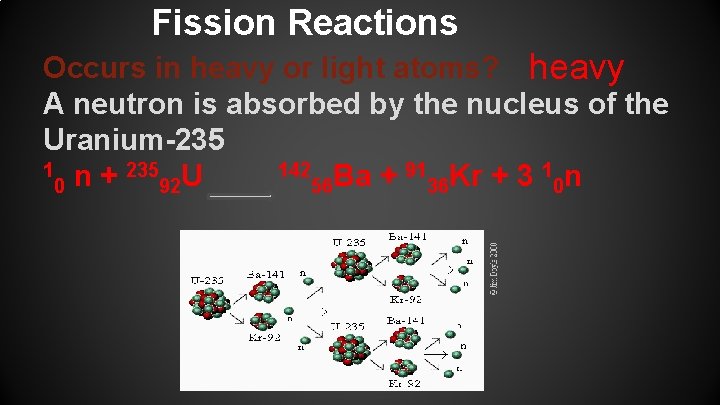
Fission Reactions Occurs in heavy or light atoms? heavy A neutron is absorbed by the nucleus of the Uranium-235 1 n + 235 U 142 Ba + 91 Kr + 3 1 n 0 92 56 36 0
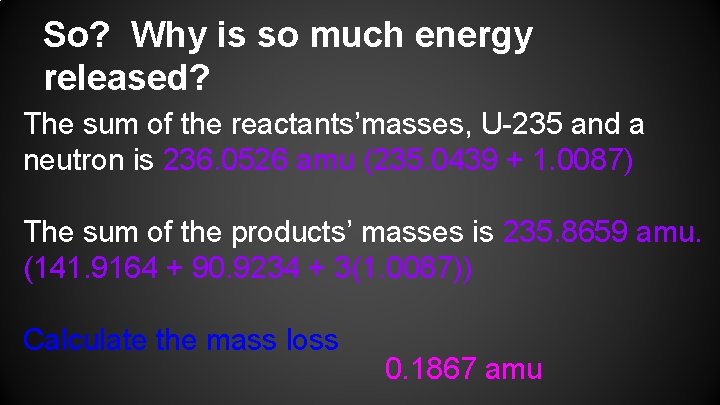
So? Why is so much energy released? The sum of the reactants’masses, U-235 and a neutron is 236. 0526 amu (235. 0439 + 1. 0087) The sum of the products’ masses is 235. 8659 amu. (141. 9164 + 90. 9234 + 3(1. 0087)) Calculate the mass loss 0. 1867 amu

Let’s use Einstein’s equation E=mc 2 0. 1867 x 1 gram x 1 kg 1 6. 022 e 23 103 g=3. 100 x 10 -28 kg E=3. 100 x 10 -28(3. 00 x 108 m/s)2 2. 79 x 10 -11 J/atom wow!

Critical Mass: When enough material, ie. U-235, is present to allow the produced neutrons to keep striking other U-235 atoms. Subcritical: When there is not enough U-235 & the neutrons then escape. Supercritical: Too much U-235 & can’t stop the reaction!

1 st Atomic Bomb: Hiroshima, Japan August 6, 1945 Two subcritical masses of U-235 slammed together to reach supercritical mass E = 20, 000 tons of TNT Result? = 8. 368 x 1013 J

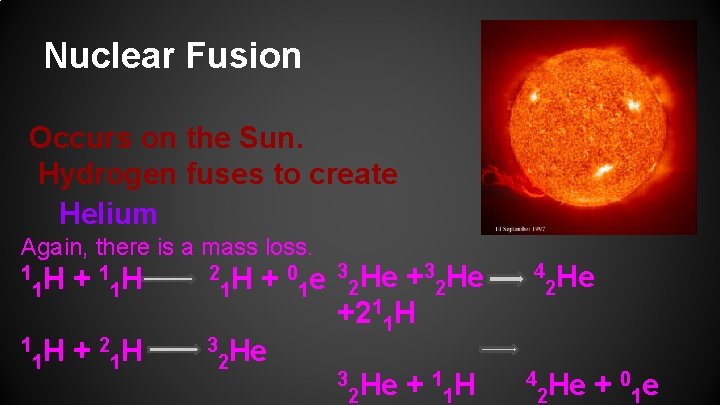
Nuclear Fusion Occurs on the Sun. Hydrogen fuses to create Helium Again, there is a mass loss. 1 1 H H + 1 1 2 H H + 1 1 3 0 e H + 1 1 2 He 3 He He + 2 2 +211 H 3 3 1 H He + 2 1 4 4 2 He 0 e He + 2 1

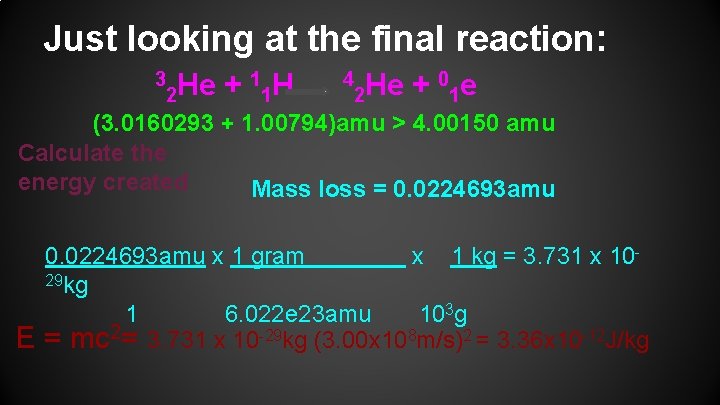
Just looking at the final reaction: 3 1 H He + 2 1 4 0 e He + 2 1 (3. 0160293 + 1. 00794)amu > 4. 00150 amu Calculate the energy created Mass loss = 0. 0224693 amu E 0. 0224693 amu x 1 gram x 1 kg = 3. 731 x 1029 kg 1 6. 022 e 23 amu 103 g = mc 2= 3. 731 x 10 -29 kg (3. 00 x 108 m/s)2 = 3. 36 x 10 -12 J/kg

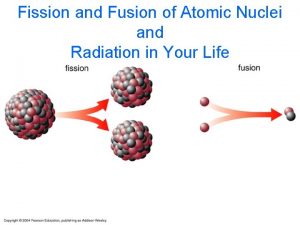
Fission v Fusion Fission: Heavy atoms that are unstable. Radioactive waste w/ long ½ life. One 7 g U-235 pellet can fuel a US house for 1. 8 months. Fusion: Light atoms that are more stable. Requires very hot temperatures. Not much radioactive waste is

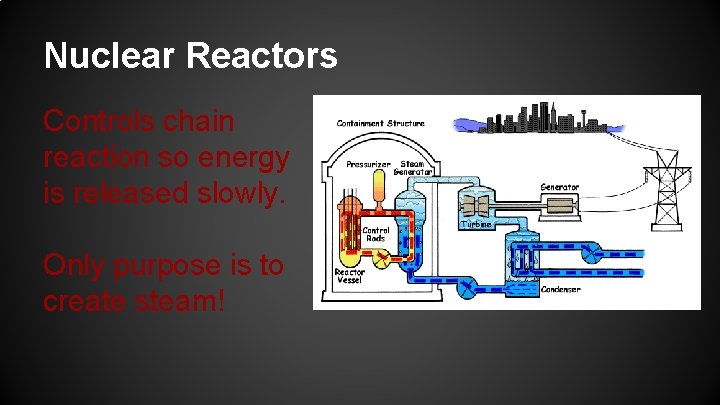
Nuclear Reactors Controls chain reaction so energy is released slowly. Only purpose is to create steam!

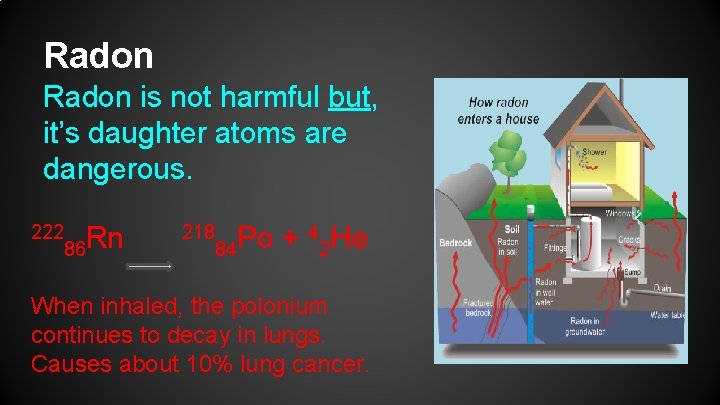
Radon is not harmful but, it’s daughter atoms are dangerous. 222 86 Rn 218 4 He Po + 84 2 When inhaled, the polonium continues to decay in lungs. Causes about 10% lung cancer.

Radiocarbon Dating Living things take in CO 2 Carbon’s natural isotopes are C-12 (99%) & C-14 (1%) When something dies, the C-14 continues to decay. 14 C 14 N + 0 ϐ 6 7 -1 C-14 half life is 5715 years. If the ratio of C-12 to C-14 is 99. 5 to. 5, then how old is the artifact? 5715 years If the ratio is 99. 75 to. 25, then how old is it? 11, 430 years.

Review of History of Atom Development Democritus (460 -370 BC) 1 st to suggest atoms are indivisible.

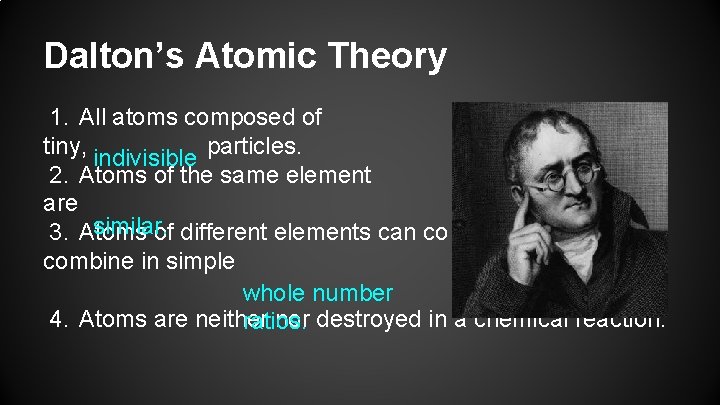
Dalton’s Atomic Theory 1. All atoms composed of tiny, indivisible particles. 2. Atoms of the same element are similar. 3. Atoms of different elements can co combine in simple whole number 4. Atoms are neither nor destroyed in a chemical reaction. ratios.

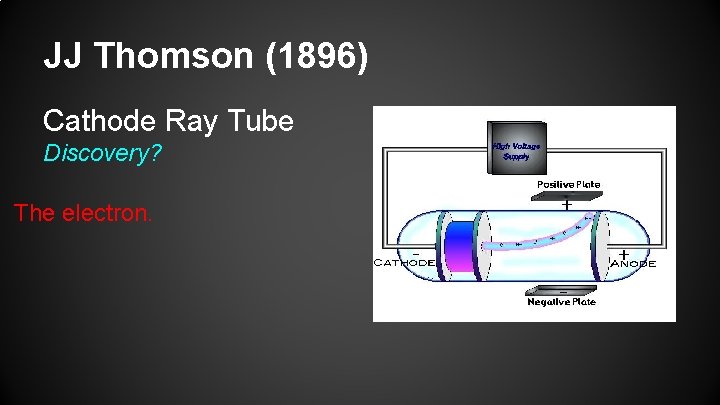
JJ Thomson (1896) Cathode Ray Tube Discovery? The electron.
 Fission vs fusion energy output
Fission vs fusion energy output Nuclear fission and fusion similarities
Nuclear fission and fusion similarities Fission v fusion
Fission v fusion Are nuclear power plants fission or fusion
Are nuclear power plants fission or fusion Fission and fusion similarities
Fission and fusion similarities Sun fusion or fission
Sun fusion or fission Fission and fusion similarities
Fission and fusion similarities Fission vs fusion
Fission vs fusion Fission vs fusion nuclear
Fission vs fusion nuclear Rds 37 bomb
Rds 37 bomb Fission and fusion similarities
Fission and fusion similarities Fusion or fission
Fusion or fission Fission vs fusion
Fission vs fusion Fusion or fission
Fusion or fission Why why why why
Why why why why Section 2 classifying chemical reactions
Section 2 classifying chemical reactions