Excited State and Ground State You know whats








- Slides: 16


Excited State and Ground State

You know what’s nice? Fireworks • Let’s watch some! • https: //www. youtube. com/watch? v=CNjggrx. UQ 78

“Ms. Wiswall, why did you have us watch a video about fireworks? ” • The way electrons move in an element is how these fireworks are produced in the first place • I will get into more details with this as we go further into the topic

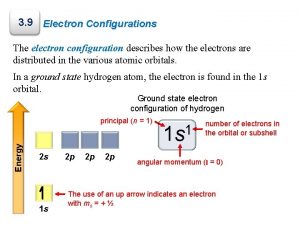
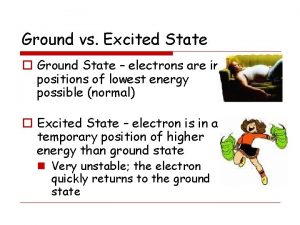
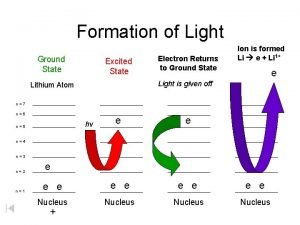
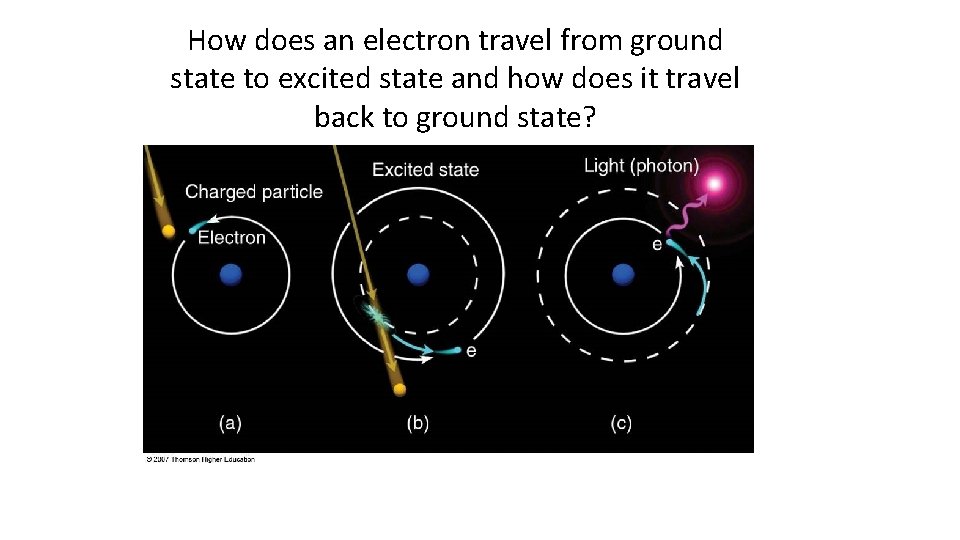
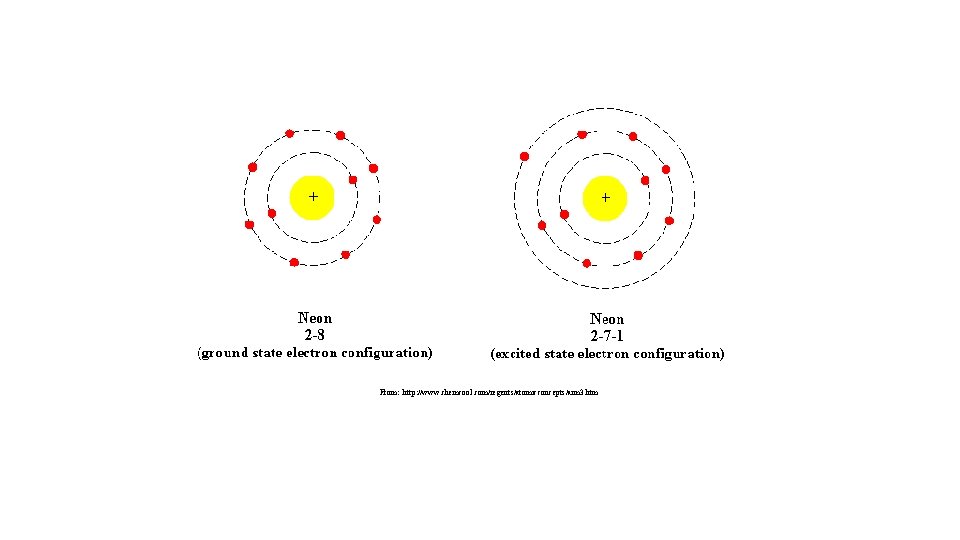
VII. Ground versus Excited State • Electrons have the ability to move between energy levels. • The “ground state” refers the energy level that a given electron usually belongs to. • The “excited state” refers to a higher energy level that a given electron moves to as it gains energy. • Electrons that have gained energy and “jumped” to the excited state will eventually return to the ground state and release its energy in the form of a photon (light energy). • As a result of this process, different atoms will give off (emit) unique (characteristic) colors (bands) of light. This is called the Bright Line Spectrum.

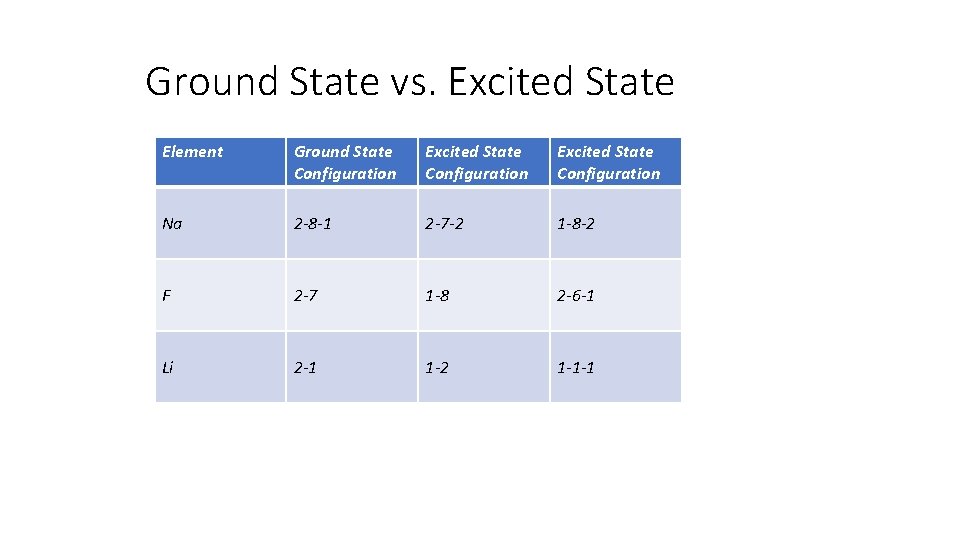
Ground State vs. Excited State Element Ground State Configuration Excited State Configuration Na 2 -8 -1 2 -7 -2 1 -8 -2 F 2 -7 1 -8 2 -6 -1 Li 2 -1 1 -2 1 -1 -1

How does an electron travel from ground state to excited state and how does it travel back to ground state?


From: http: //www. chemcool. com/regents/atomicconcepts/aim 3. htm

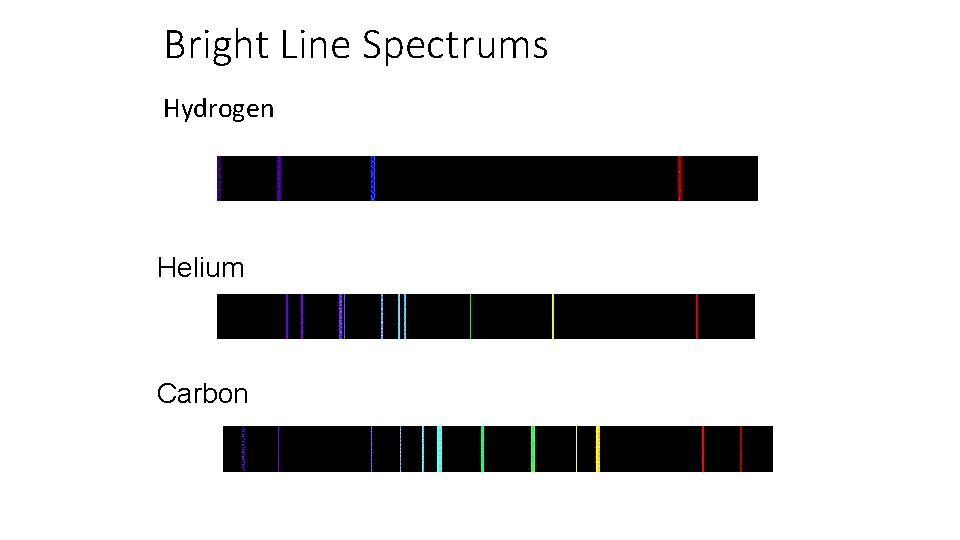
Bright Line Spectrums Hydrogen Helium Carbon

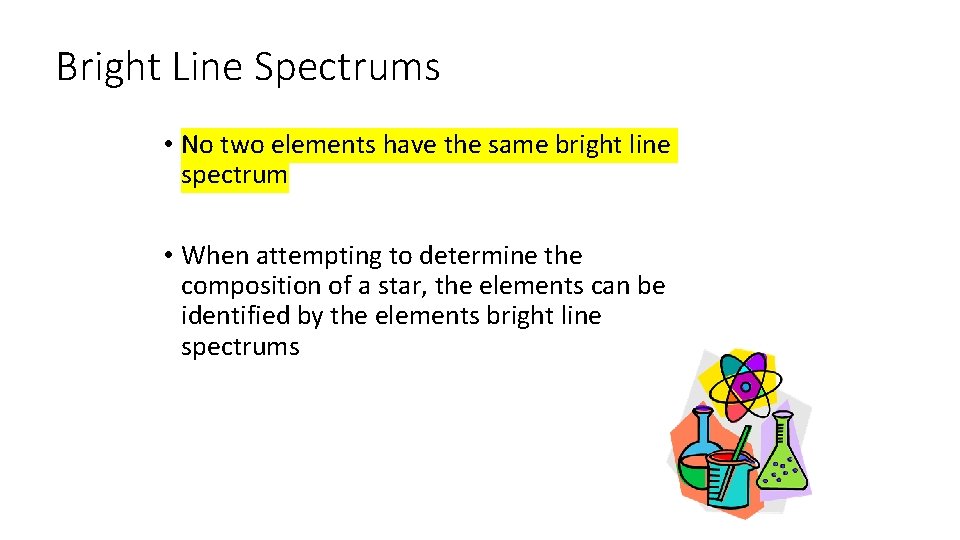
Bright Line Spectrums • No two elements have the same bright line spectrum • When attempting to determine the composition of a star, the elements can be identified by the elements bright line spectrums

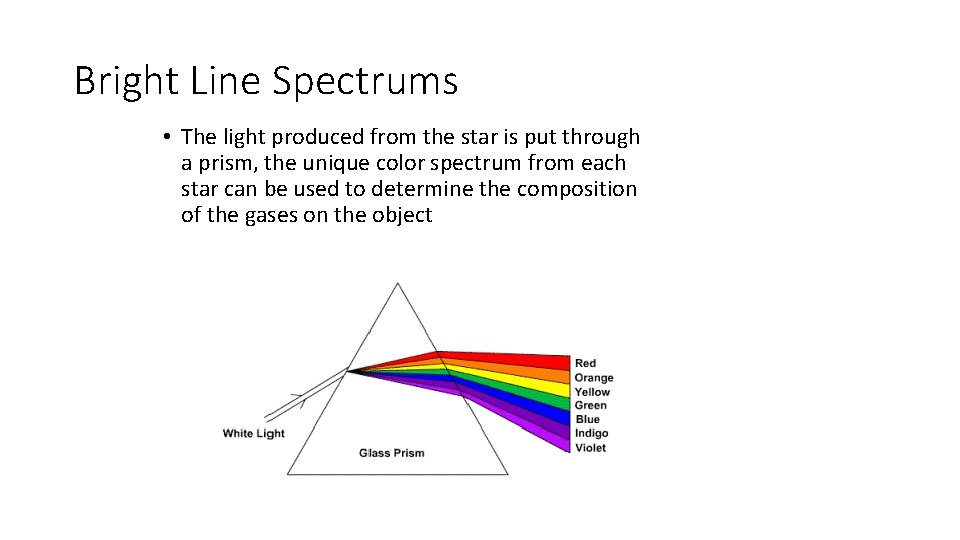
Bright Line Spectrums • The light produced from the star is put through a prism, the unique color spectrum from each star can be used to determine the composition of the gases on the object

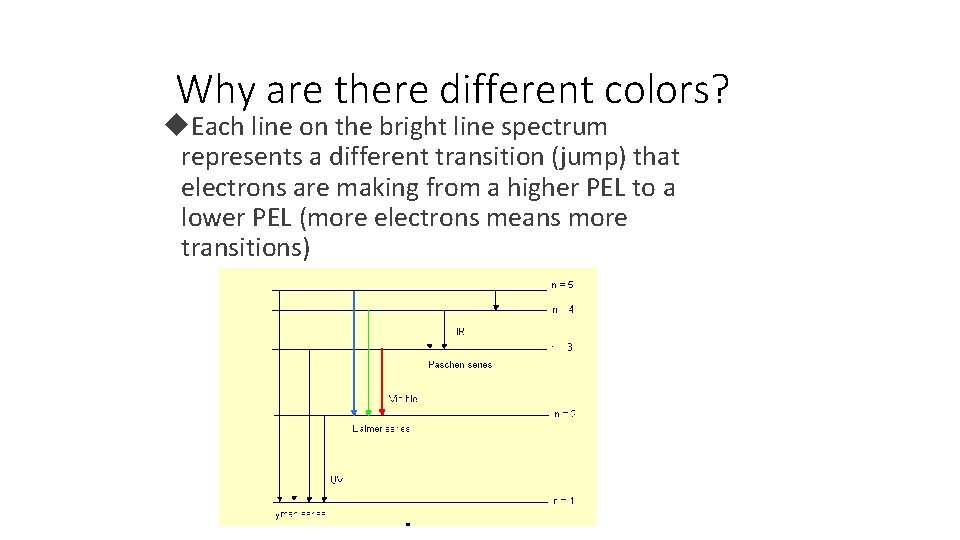
Why are there different colors? Each line on the bright line spectrum represents a different transition (jump) that electrons are making from a higher PEL to a lower PEL (more electrons means more transitions)

When do we see this? • We can create bright line spectrums by running an electric current through a gas • The electric current gives the electrons more energy letting them jump to an excited state, and producing light when they fall back down to ground state

Base your answers to the following questions on the diagram below, which shows bright-line spectra of selected elements. • Identify the two elements in the unknown spectrum. • Explain how a bright-line spectrum is produced, in terms of excited state and ground state.

Let’s get back into fireworks • As you can see in this image, each firework color is actually made by a certain element who’s electrons are going from excited to ground state.
 Shell vs subshell
Shell vs subshell Ground vs excited state
Ground vs excited state Speech good morning everyone
Speech good morning everyone The most helpful classmates are the ones who
The most helpful classmates are the ones who If you are happy
If you are happy Personification in the raven
Personification in the raven When you're blue and you don't know
When you're blue and you don't know Know history know self
Know history know self Dilan gorur
Dilan gorur I'm holding on to your promises you are faithful
I'm holding on to your promises you are faithful Excited state electron configuration
Excited state electron configuration Bohr atom model
Bohr atom model Ground state of neon
Ground state of neon Excited state electron configuration
Excited state electron configuration Fact about minecraft
Fact about minecraft Am i asexual
Am i asexual Do you know who you are
Do you know who you are