Evaluating Vaccine Uptake and Safety Using Immunization Information












- Slides: 24

Evaluating Vaccine Uptake and Safety Using Immunization Information Systems and VAERS CDC STD Prevention Conference Diana Bartlett, Laura Williams, and Robin Curtis Immunization Services Division NCIRD/CDC The findings and conclusions in this presentation have not been formally disseminated by CDC and should not be construed to represent any agency determination or policy.

Outline § Overview of immunization information § § § systems (IIS) IIS Sentinel Site project – Description – Preliminary data Other assessment methods: – National Immunization Survey (NIS)-Teen – NIS-Adult Safety monitoring: Vaccine Safety Datalink and Vaccine Adverse Event System (VAERS)

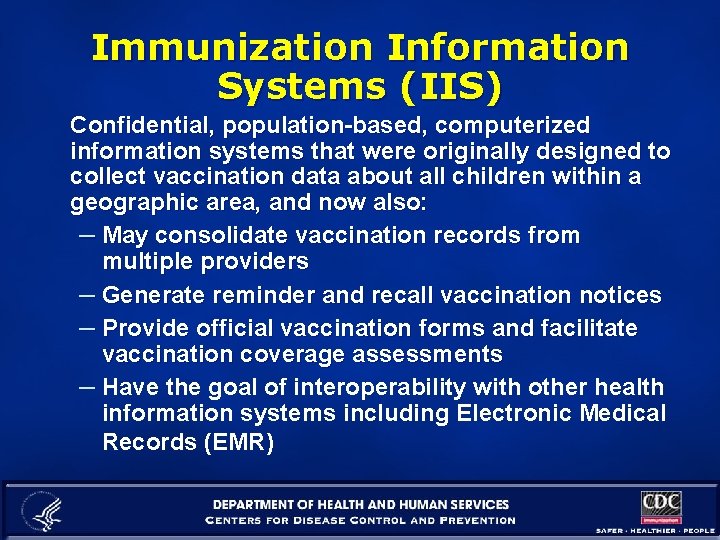
Immunization Information Systems (IIS) Confidential, population-based, computerized information systems that were originally designed to collect vaccination data about all children within a geographic area, and now also: – May consolidate vaccination records from multiple providers – Generate reminder and recall vaccination notices – Provide official vaccination forms and facilitate vaccination coverage assessments – Have the goal of interoperability with other health information systems including Electronic Medical Records (EMR)

Age groups participating in an IIS Source: 2006 Immunization Information System Annual Report

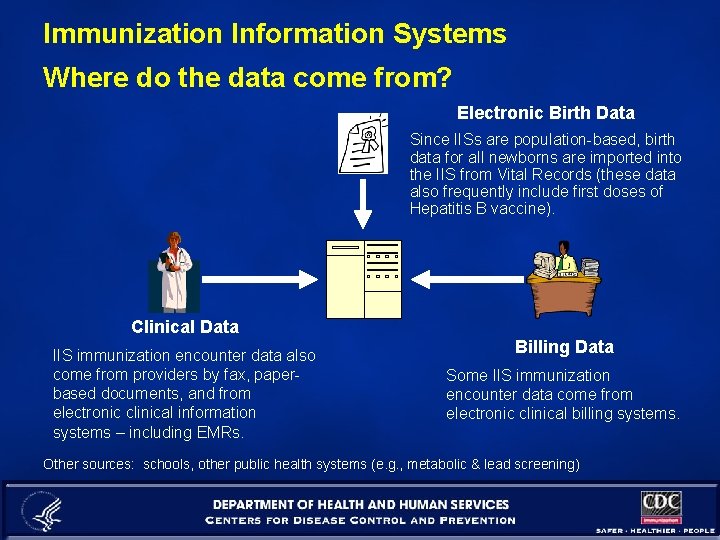
Immunization Information Systems Where do the data come from? Electronic Birth Data Since IISs are population-based, birth data for all newborns are imported into the IIS from Vital Records (these data also frequently include first doses of Hepatitis B vaccine). Clinical Data IIS immunization encounter data also come from providers by fax, paperbased documents, and from electronic clinical information systems – including EMRs. Billing Data Some IIS immunization encounter data come from electronic clinical billing systems. Other sources: schools, other public health systems (e. g. , metabolic & lead screening)

IIS in the U. S. • CDC provides annual funding to 56 Immunization Program Grantees (50 states, Washington DC, and 5 large cities) • IIS development and implementation are a required activity component for immunization programs • IIS core data set fields include name, date of birth, race/ethnicity, gender, vaccine information, provider who administered the dose, and eligibility for VFC • 8 grantees receive supplemental funds to serve as Sentinel Sites for IIS-based evaluation projects

Using IIS for Vaccine Coverage Assessments: Strengths & Limitations § Strengths – Can provide valid, timely and detailed geographic coverage estimates – May include information from multiple providers § Limitations – Provider participation may not be sufficiently high in areas of interest and may not fully reflect all types of providers administering vaccines – Estimates may be affected by enrollees’ mobility within state & in/out state – Data may not be adequately de-duplicated (e. g. , multiple records may exist for one child) – Systems may not yet completely represent all age groups of interest

IIS Sentinel Sites Project, 2004 -2007 § Assesses coverage for persons aged <19 years § Requires and employs enhanced data quality procedures § Includes IIS subsets in AZ, MI, MN, MT, OR , and DC: – Representing contiguous geographic counties, postal code areas, or census tracts – Having high (~90%) healthcare provider site participation and enrollment of children in each IIS

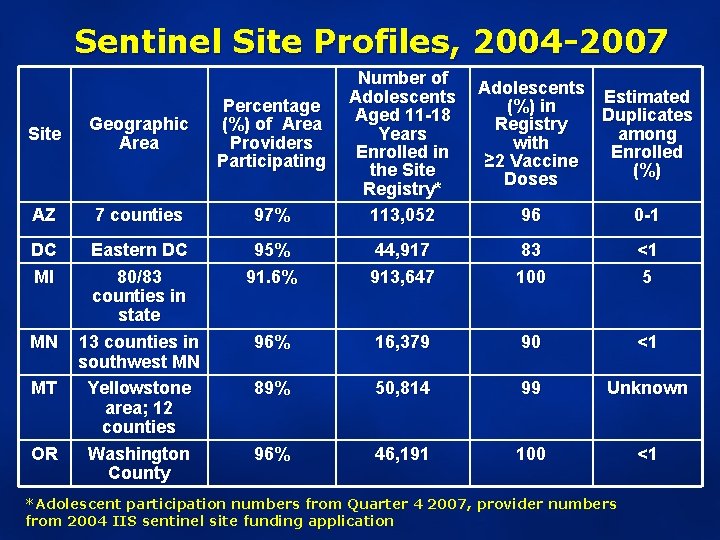
Sentinel Site Profiles, 2004 -2007 Site Geographic Area Percentage (%) of Area Providers Participating AZ 7 counties 97% Number of Adolescents Aged 11 -18 Years Enrolled in the Site Registry* 113, 052 DC MI Eastern DC 80/83 counties in state 13 counties in southwest MN Yellowstone area; 12 counties Washington County 95% 91. 6% MN MT OR Adolescents Estimated (%) in Duplicates Registry among with Enrolled ≥ 2 Vaccine (%) Doses 96 0 -1 44, 917 913, 647 83 100 <1 5 96% 16, 379 90 <1 89% 50, 814 99 Unknown 96% 46, 191 100 <1 *Adolescent participation numbers from Quarter 4 2007, provider numbers from 2004 IIS sentinel site funding application

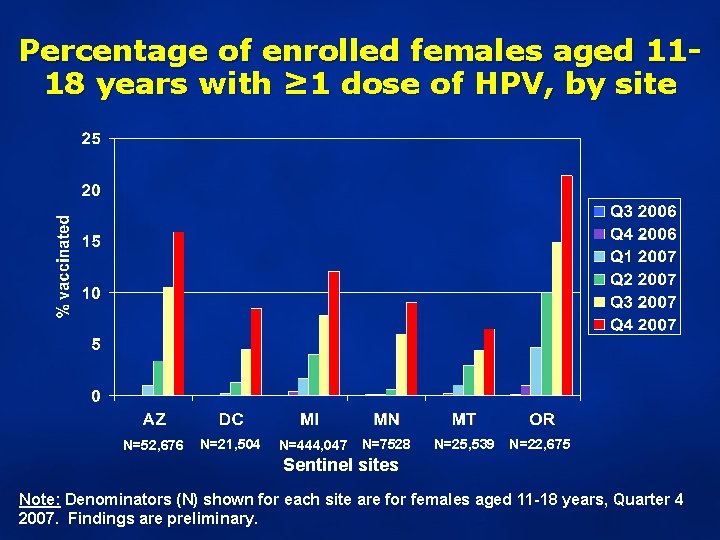
Percentage of enrolled females aged 1118 years with ≥ 1 dose of HPV, by site N=52, 676 N=21, 504 N=444, 047 N=7528 N=25, 539 N=22, 675 Sentinel sites Note: Denominators (N) shown for each site are for females aged 11 -18 years, Quarter 4 2007. Findings are preliminary.

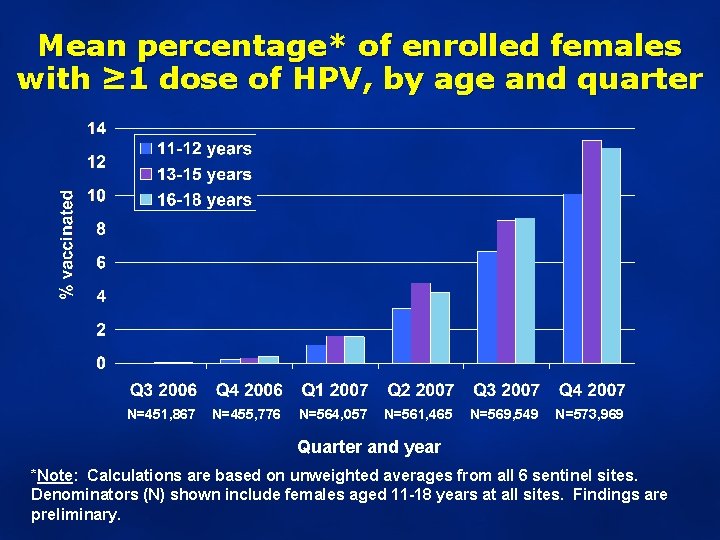
Mean percentage* of enrolled females with ≥ 1 dose of HPV, by age and quarter N=451, 867 N=455, 776 N=564, 057 N=561, 465 N=569, 549 N=573, 969 Quarter and year *Note: Calculations are based on unweighted averages from all 6 sentinel sites. Denominators (N) shown include females aged 11 -18 years at all sites. Findings are preliminary.

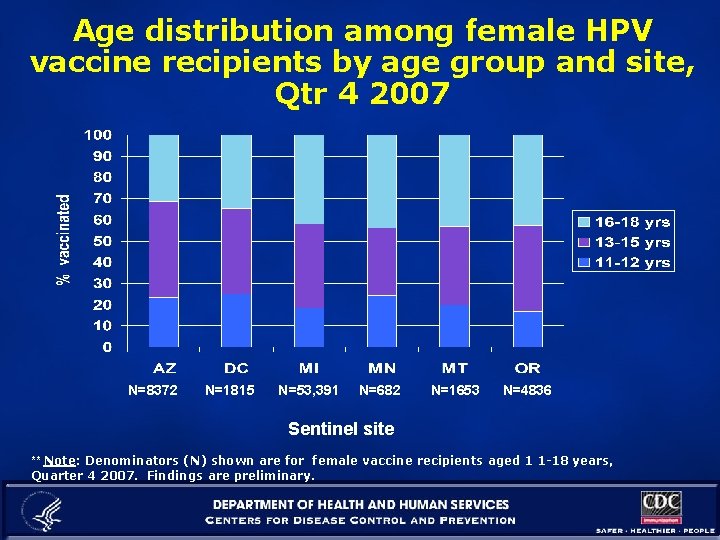
Age distribution among female HPV vaccine recipients by age group and site, Qtr 4 2007 N=8372 N=1815 N=53, 391 N=682 N=1653 N=4836 Sentinel site ** Note: Denominators (N) shown are for female vaccine recipients aged 1 1 -18 years, Quarter 4 2007. Findings are preliminary.

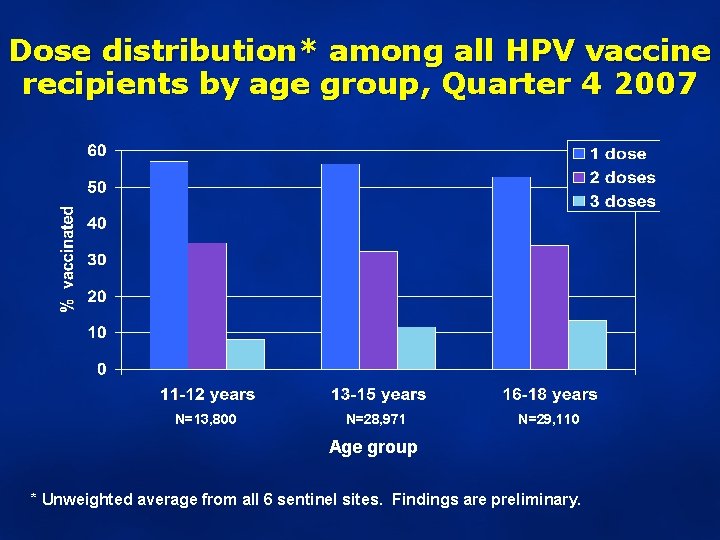
Dose distribution* among all HPV vaccine recipients by age group, Quarter 4 2007 N=13, 800 N=28, 971 N=29, 110 Age group * Unweighted average from all 6 sentinel sites. Findings are preliminary.

National Immunization Surveys § Established survey among children aged 19 -35 months currently focuses on 50 states and six urban areas directly receiving federal immunization grant funds – State immunization grantees can choose city/county areas to include in the NIS using their grant funds § NIS-Teen administered in 4 th quarters 2006 and 2007 among persons aged 13 -17 years (national data only) § NIS-Adult administered May-August 2007 for persons aged ≥ 18 years (national data, not record validated)

Next Steps § IIS Sentinel Site project will continue to monitor HPV vaccine uptake § CDC has added vaccine receipt questions to the NHIS, BRFSS, NSCH, and other surveys § The NIS-Teen has been expanded to state/grantee level in 2008 and will continue annually

VAERS § National post-licensure safety surveillance system jointly operated by CDC and FDA § Spontaneous reporting system in existence since 1990; reports submitted by clinicians, manufacturers, patients/parents, others § Subject to well described limitations of passive surveillance including underreporting, stimulated reporting due to media attention and other factors, and lack of availability of denominator data


Acknowledgments § Sentinel Site Colleagues: – Arizona: Lisa Rasmussen and Kathy Fredrickson – Washington, DC: Rosie Mc. Laren and Cherie Thomas – Michigan: Kyle Enger and Laura Rappleye – Minnesota: Karen White and Emily Peterson-Stauffer – Montana: Bekki Kirsch and Joyce Burgett – Oregon: Jim Gaudino and Heather Crawford § National Center for HIV, STD, and Tuberculosis Prevention: Lauri Markowitz § National Center for Immunization and Respiratory Diseases: Sharad Aggarwal, Laura Williams, Angela Calugar, Robin Curtis, Gary Urquhart

For More Information § Contact: – Diana Bartlett: dbartlett@cdc. gov Thank you!

Extra slides (as needed)

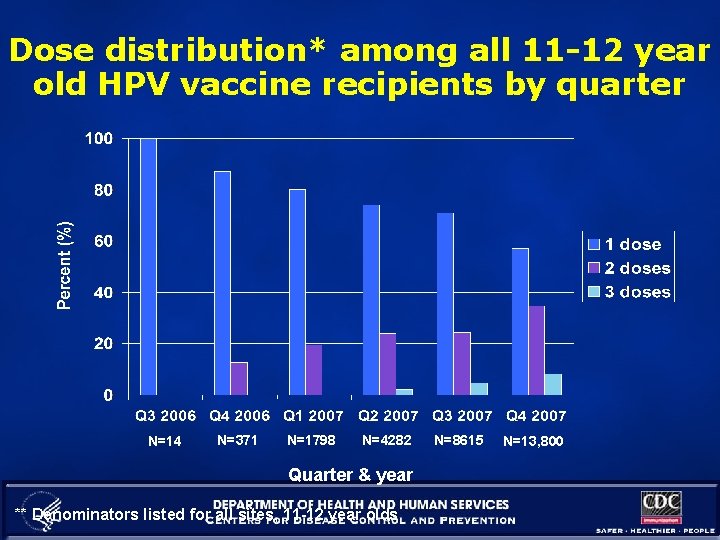
Dose distribution* among all 11 -12 year old HPV vaccine recipients by quarter N=14 N=371 N=1798 N=4282 Quarter & year ** Denominators listed for all sites, 11 -12 year olds N=8615 N=13, 800

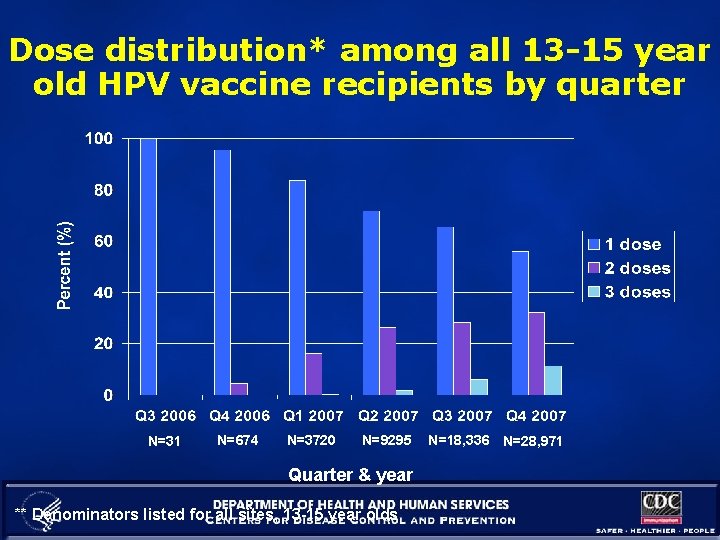
Dose distribution* among all 13 -15 year old HPV vaccine recipients by quarter N=31 N=674 N=3720 N=9295 Quarter & year ** Denominators listed for all sites, 13 -15 year olds N=18, 336 N=28, 971

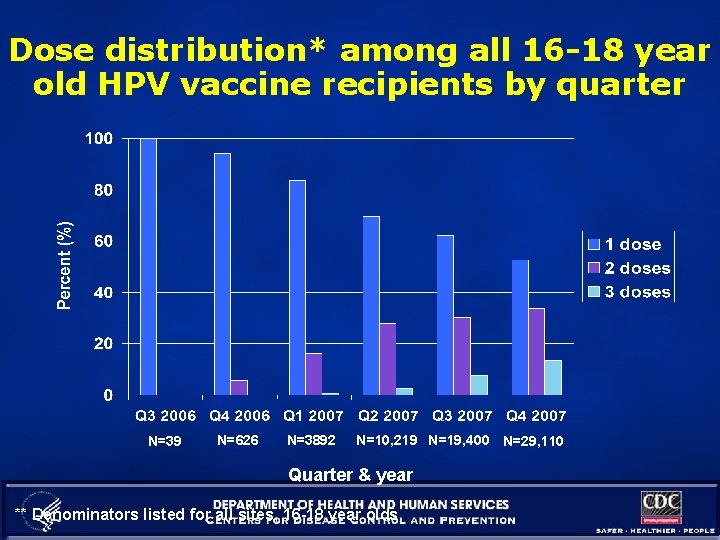
Dose distribution* among all 16 -18 year old HPV vaccine recipients by quarter N=39 N=626 N=3892 N=10, 219 N=19, 400 N=29, 110 Quarter & year ** Denominators listed for all sites, 16 -18 year olds

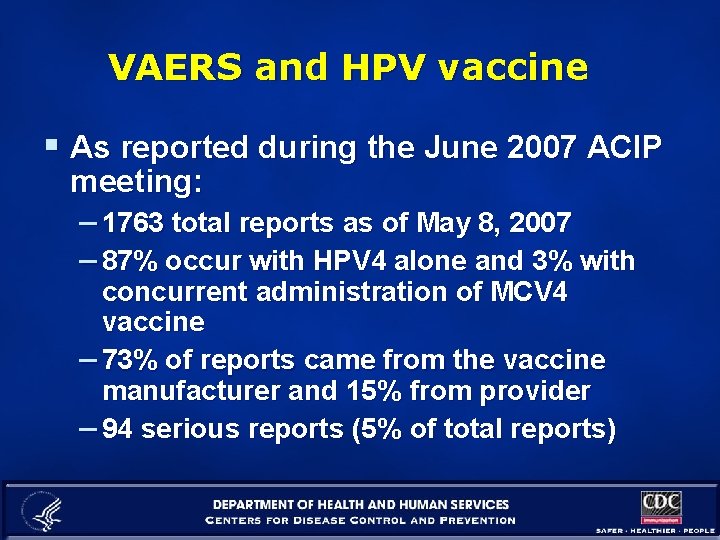
VAERS and HPV vaccine § As reported during the June 2007 ACIP meeting: – 1763 total reports as of May 8, 2007 – 87% occur with HPV 4 alone and 3% with concurrent administration of MCV 4 vaccine – 73% of reports came from the vaccine manufacturer and 15% from provider – 94 serious reports (5% of total reports)