Uptake of Rotavirus Vaccine and Adherence with ACIP







- Slides: 19

Uptake of Rotavirus Vaccine and Adherence with ACIP Age Recommendations Haley A. Clayton, MPH Centers for Disease Control and Prevention March 17, 2008 9/19/2021

Rotavirus Vaccine Pentavalent (Rota. Teq®) Recommended by ACIP February 2006 – Three doses: ages 2, 4, 6 months Dose 1: age 6 -12 weeks Subsequent doses 4 -10 weeks apart No doses after 32 weeks of age

Rota. Teq® schedule Age recommendations consistent with prelicensure clinical trials Previous rotavirus vaccine, Rota. Shield® removed from the market association with intussusception greatest risk noted after first dose

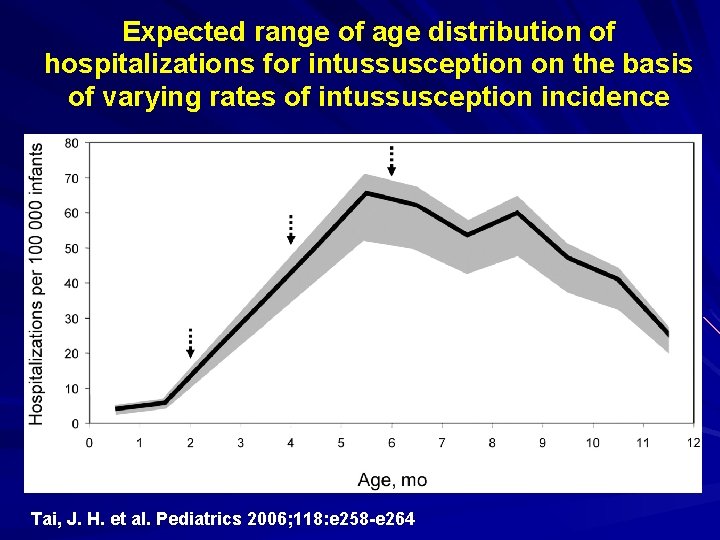
Expected range of age distribution of hospitalizations for intussusception on the basis of varying rates of intussusception incidence Tai, J. H. et al. Pediatrics 2006; 118: e 258 -e 264

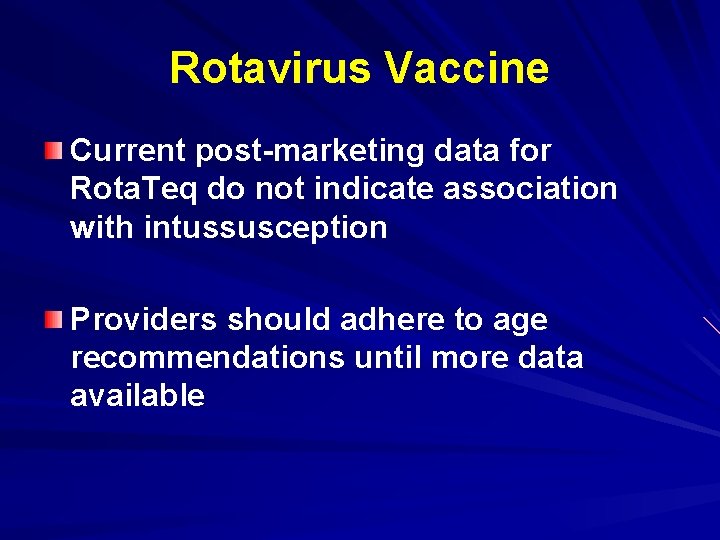
Rotavirus Vaccine Current post-marketing data for Rota. Teq do not indicate association with intussusception Providers should adhere to age recommendations until more data available

Aims of Analysis To assess: 1. Rotavirus vaccine uptake 2. Rotavirus vaccine coverage 3. Adherence to rotavirus vaccine age recommendations

Data Sources Immunization Information Systems (IIS) Confidential, computerized records of vaccine administration for a geographic area In 2006, approximately 65% of U. S. children <6 years of age participated in an IIS Sentinel Sites Population-based subsets of the state IIS coverage area >10, 000 children <6 years of age, > 90% participation Procedures to increase completeness and accuracy Vaccine Safety Datalink (VSD) Collaborative project with CDC and 8 medical care organizations >5. 5 million people annually, comprehensive immunization histories Routine data quality checks

Results of Analysis

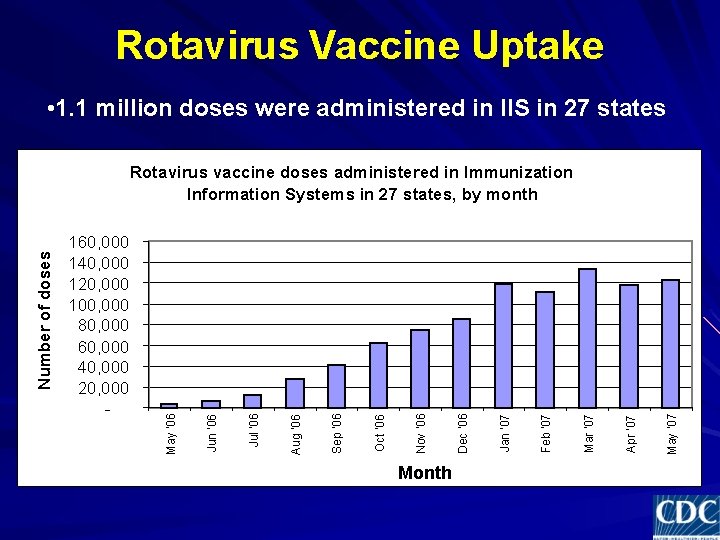
Rotavirus Vaccine Uptake • 1. 1 million doses were administered in IIS in 27 states Month May '07 Apr '07 Mar '07 Feb '07 Jan '07 Dec '06 Nov '06 Oct '06 Sep '06 Aug '06 Jul '06 Jun '06 160, 000 140, 000 120, 000 100, 000 80, 000 60, 000 40, 000 20, 000 - May '06 Number of doses Rotavirus vaccine doses administered in Immunization Information Systems in 27 states, by month

Rotavirus Vaccine Coverage First dose rotavirus vaccination coverage among children aged 3 months in IIS Sentinel Sites, by quarter and site % Vaccinated 100 80 Q 3 2006 Q 4 2006 Q 1 2007 Q 2 2007 Q 3 2007 Q 4 2007 60 40 20 0 Arizona DC Michigan Minnesota Montana Sentinel Site Oregon

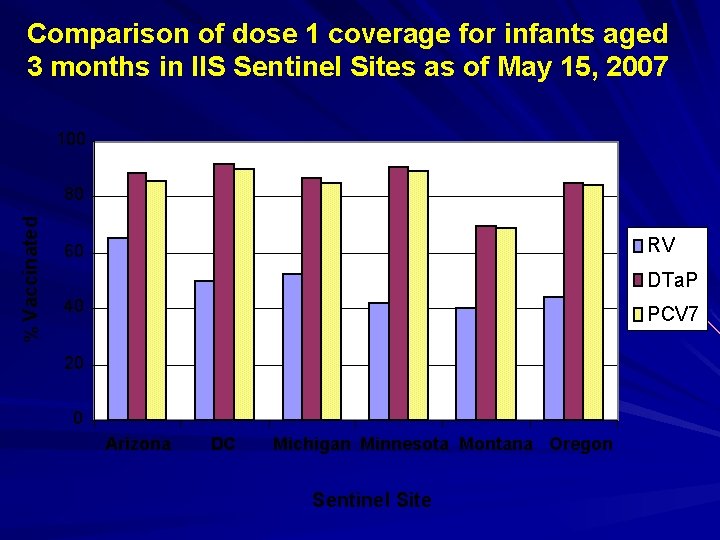
Comparison of dose 1 coverage for infants aged 3 months in IIS Sentinel Sites as of May 15, 2007 100 % Vaccinated 80 RV 60 DTa. P 40 PCV 7 20 0 Arizona DC Michigan Minnesota Montana Oregon Sentinel Site

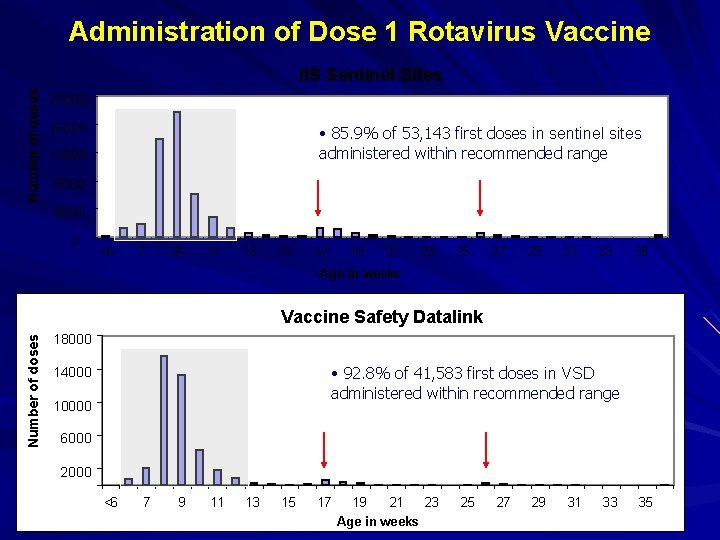
Assessment of Age Adherence Data reported by week of age at administration and dose number in series IIS sentinel sites VSD Date of vaccine administration determined dose number in the series First date of vaccine administration counting as dose 1

Administration of Dose 1 Rotavirus Vaccine Number of doses IIS Sentinel Sites 20000 16000 • 85. 9% of 53, 143 first doses in sentinel sites administered within recommended range 12000 8000 4000 0 <6 7 9 11 13 15 17 19 21 23 25 27 29 31 33 35 Age in weeks Number of doses Vaccine Safety Datalink 18000 • 92. 8% of 41, 583 first doses in VSD administered within recommended range 14000 10000 6000 2000 <6 7 9 11 13 15 17 19 21 Age in weeks 23 25 27 29 31 33 35

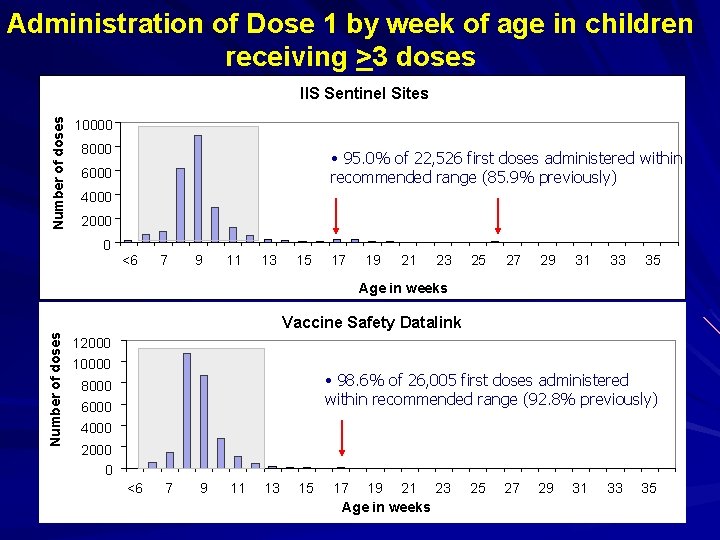
Assessment of Age Adherence Are apparent first doses given at 17 and 26 weeks really first doses? – Date of vaccination used to determine dose number in the series Possibility they are 2 nd or 3 rd doses recorded as first dose – Ages coincide with timing of 2 nd and 3 rd doses of infant vaccines, including rotavirus vaccine Decided to look at only children with 3 doses recorded

Administration of Dose 1 by week of age in children receiving >3 doses Number of doses IIS Sentinel Sites 10000 8000 • 95. 0% of 22, 526 first doses administered within recommended range (85. 9% previously) 6000 4000 2000 0 <6 7 9 11 13 15 17 19 21 23 25 27 29 31 33 35 Age in weeks Number of doses Vaccine Safety Datalink 12000 10000 • 98. 6% of 26, 005 first doses administered within recommended range (92. 8% previously) 8000 6000 4000 2000 0 <6 7 9 11 13 15 17 19 21 23 Age in weeks 25 27 29 31 33 35

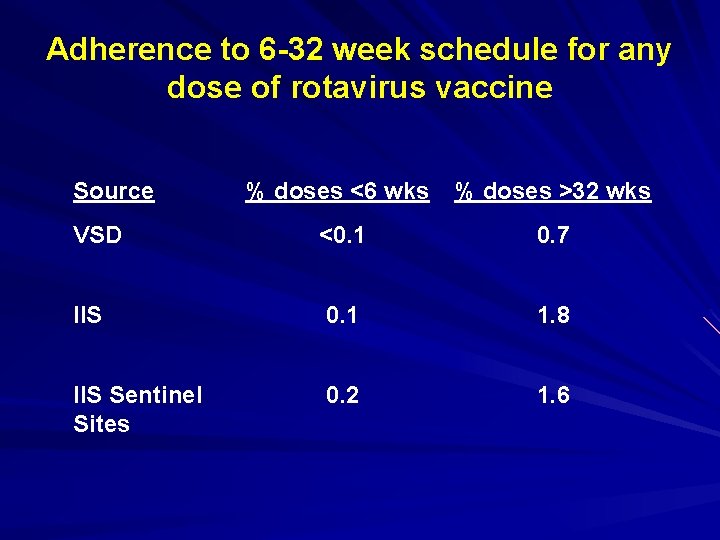
Adherence to 6 -32 week schedule for any dose of rotavirus vaccine Source % doses <6 wks % doses >32 wks VSD <0. 1 0. 7 IIS 0. 1 1. 8 IIS Sentinel Sites 0. 2 1. 6

Limitations Results may not be nationally representative Likely that vaccinations missing from children’s electronic records underestimation of vaccination coverage levels

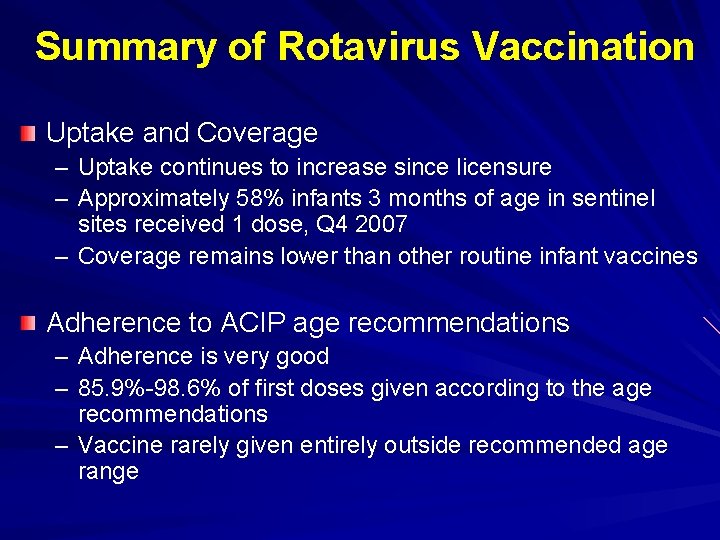
Summary of Rotavirus Vaccination Uptake and Coverage – Uptake continues to increase since licensure – Approximately 58% infants 3 months of age in sentinel sites received 1 dose, Q 4 2007 – Coverage remains lower than other routine infant vaccines Adherence to ACIP age recommendations – Adherence is very good – 85. 9%-98. 6% of first doses given according to the age recommendations – Vaccine rarely given entirely outside recommended age range

Acknowledgments NCIRD – – – Margaret Cortese Dan Payne Umesh Parashar Lauren Stockman Minnie Wang ISD – Diana Bartlett – Laura Zimmerman – Warren Williams ISO: VSD – – James Baggs Paul Gargiullo Eric Weintraub Sites participating in VSD State Immunization Information Systems Sentinel Area Immunization Information Systems Ø AZ: Lisa Rasmussen and Kathy Fredrickson Ø DC: Rosie Mc. Laren and Cherie Thomas Ø MI: Kyle Enger and Laura Rappleye Ø MN: Karen White and Emily Peterson-Stauffer Ø MT: Bekki Wehner and Joyce Burgett Ø OR: Jim Gaudino and Heather Crawford
 Physiological adaptations pdhpe
Physiological adaptations pdhpe Cholesterol uptake
Cholesterol uptake At the same oxygen uptake, arm work results in _____.
At the same oxygen uptake, arm work results in _____. Hemoglobn
Hemoglobn Pdt rokotus
Pdt rokotus Reovirus
Reovirus Psvi rotavirus
Psvi rotavirus Rotavirus
Rotavirus Rotavirus
Rotavirus Rotavirus
Rotavirus Rotavirus
Rotavirus Exercise behavior and adherence
Exercise behavior and adherence Adhérence préputiale traitement
Adhérence préputiale traitement Liaison démontable
Liaison démontable Liaison complète par tampons tangents
Liaison complète par tampons tangents Declakon
Declakon Cdph adherence monitoring tools
Cdph adherence monitoring tools Vaccine storage and handling sop worksheet
Vaccine storage and handling sop worksheet Vaccine storage and handling protocol
Vaccine storage and handling protocol Edible vaccine definition
Edible vaccine definition