Training for rotavirus vaccine introduction Module 6 Rotavirus





- Slides: 10

Training for rotavirus vaccine introduction Module 6 Rotavirus vaccine AEFI monitoring

Learning objectives l At the end of the module, the participants will be able to: – Identify adverse events following immunization (AEFIs), including intussusception (IS) – Explain how to report AEFIs l Duration – 15’ 2| Rotavirus vaccine AEFI monitoring, Module 6 I 03 October 2020

Key issues 1 What is an AEFI? 2 What is intussusception (IS)? 3 How to report an AEFI? 3| Rotavirus vaccine AEFI monitoring, Module 6 I 03 October 2020

What is an AEFI? l AEFI = Adverse event following immunization – – A medical incident Takes place after an immunization Causes concern Is believed to be caused by immunization l AEFI can be categorized into – – – 4| Vaccine reaction Programme error Coincidental Injection reaction Unknown Rotavirus vaccine AEFI monitoring, Module 6 I 03 October 2020

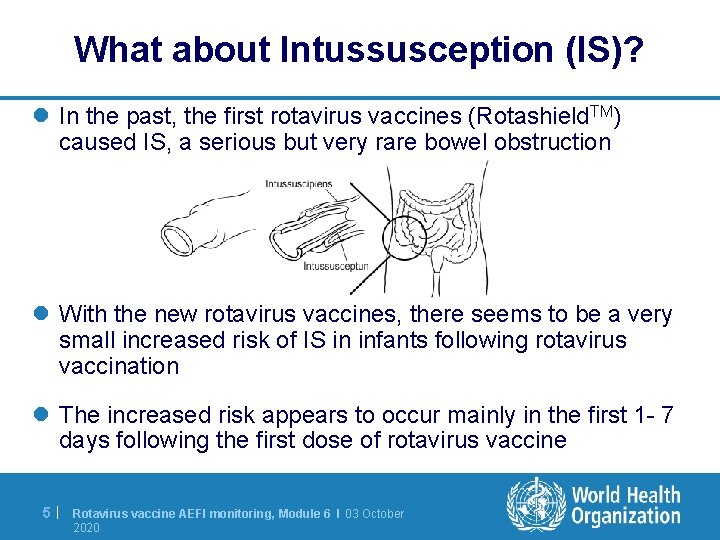
What about Intussusception (IS)? l In the past, the first rotavirus vaccines (Rotashield. TM) caused IS, a serious but very rare bowel obstruction l With the new rotavirus vaccines, there seems to be a very small increased risk of IS in infants following rotavirus vaccination l The increased risk appears to occur mainly in the first 1 - 7 days following the first dose of rotavirus vaccine 5| Rotavirus vaccine AEFI monitoring, Module 6 I 03 October 2020

Risk of IS against risk of rotavirus infection 2/2 l Whether the new rotavirus vaccine (Rotavac. TM) affects the overall incidence of IS has not yet been established l The risk of IS after rotavirus vaccination is much lower than the risk of severe rotavirus disease in unvaccinated infants and young children! 6| Rotavirus vaccine AEFI monitoring, Module 6 I 03 October 2020

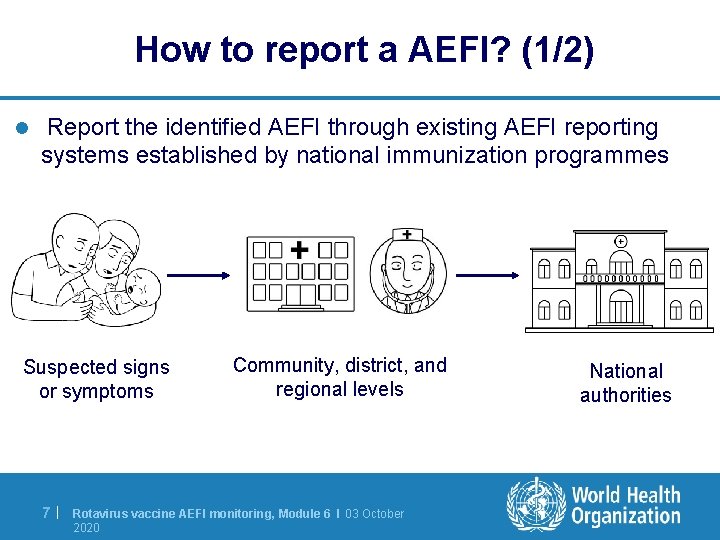
How to report a AEFI? (1/2) l Report the identified AEFI through existing AEFI reporting systems established by national immunization programmes Suspected signs or symptoms 7| Community, district, and regional levels Rotavirus vaccine AEFI monitoring, Module 6 I 03 October 2020 National authorities

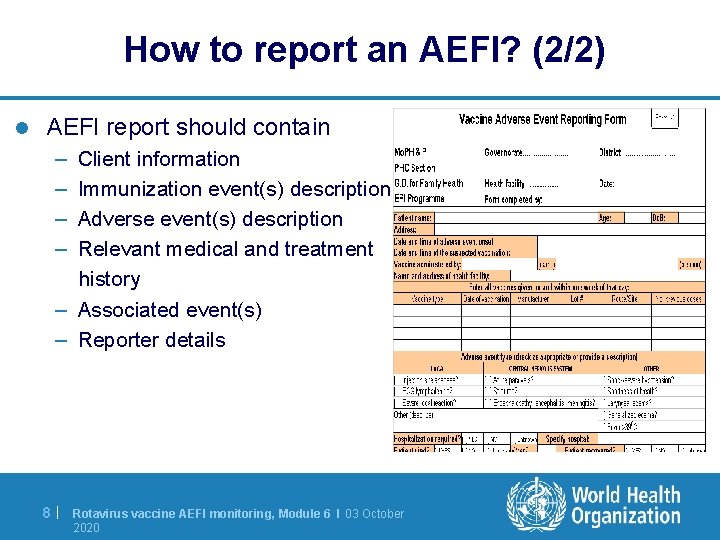
How to report an AEFI? (2/2) l AEFI report should contain – – Client information Immunization event(s) description Adverse event(s) description Relevant medical and treatment history – Associated event(s) – Reporter details 8| Rotavirus vaccine AEFI monitoring, Module 6 I 03 October 2020

Key messages l The current safety profile of rotavirus vaccines is good l Many infants who get the rotavirus vaccine (Rotavac. TM) do not experience any side effects l The risk of IS after rotavirus vaccination is much lower than the risk of severe rotavirus disease in unvaccinated infants and young children l AEFIs should be reported through the existing AEFI reporting systems/forms l Reassure the caretaker- admit uncertainty, investigate fully, and keep the community informed 9| Rotavirus vaccine AEFI monitoring, Module 6 I 03 October 2020

End of module Thank you for your attention! 10 | Rotavirus vaccine AEFI monitoring, Module 6 I 03 October 2020