Training for rotavirus vaccine introduction Module 2 Rotavirus











- Slides: 14

Training for rotavirus vaccine introduction Module 2 Rotavirus vaccine attributes and storage conditions

Learning objectives l At the end of the module, the participant will be able to: – Describe the main attributes of Rotateq. TM vaccine – Describe storage conditions of Rotateq. TM vaccine l Duration – 15’ 2 | Rotavirus vaccine attributes and storage conditions, November 2020 Module 2 | 02

Key issues 1 What is rotavirus vaccine presentation? 2 How safe is rotavirus vaccine? 3 At which temperature should the vaccine be stored? 4 Where should the vaccine be stored? 3 | Rotavirus vaccine attributes and storage conditions, November 2020 Module 2 | 02

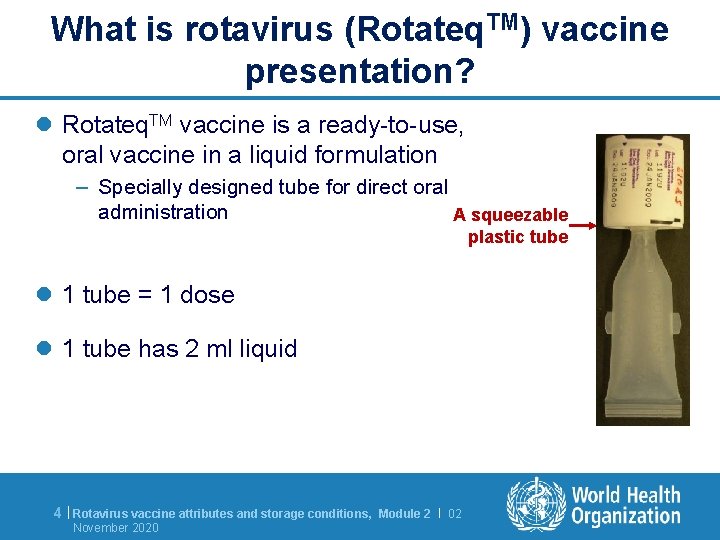
What is rotavirus (Rotateq. TM) vaccine presentation? l Rotateq. TM vaccine is a ready-to-use, oral vaccine in a liquid formulation – Specially designed tube for direct oral administration A squeezable plastic tube l 1 tube = 1 dose l 1 tube has 2 ml liquid 4 | Rotavirus vaccine attributes and storage conditions, November 2020 Module 2 | 02

How safe is the vaccine? l Rotateq. TM vaccine is safe and does NOT cause any serious adverse events l Irritability and loss of appetite are very common side effects of Rotateq. TM vaccine l Rotateq. TM vaccine may be given with other vaccines in the infant Expanded Programme on Immunization (EPI) schedule without interfering with their effectiveness 5 | Rotavirus vaccine attributes and storage conditions, November 2020 Module 2 | 02

At what temperature must the vaccine be stored? l Rotateq. TM vaccine should be stored between +2°C to +8°C l Care should be taken that the vaccine not be frozen 6 | Rotavirus vaccine attributes and storage conditions, November 2020 Module 2 | 02

Where do you store the vaccine? l The rotavirus vaccine (Rotateq. TM) should be stored in a refrigerator 7 | Rotavirus vaccine attributes and storage conditions, November 2020 Module 2 | 02

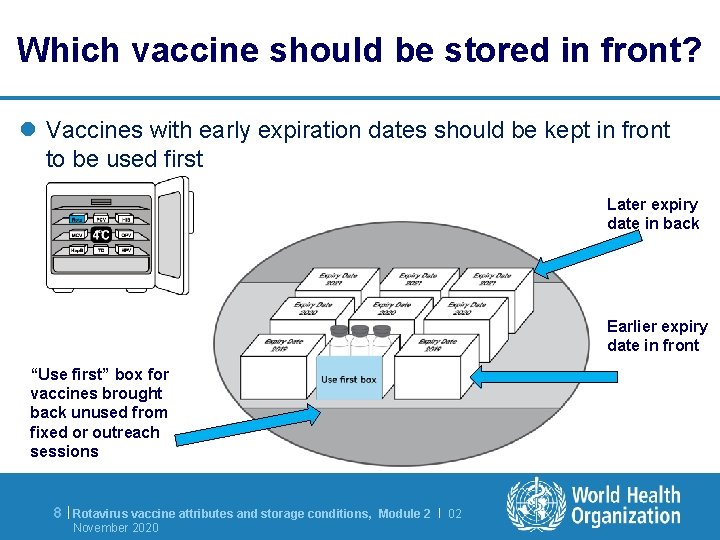
Which vaccine should be stored in front? l Vaccines with early expiration dates should be kept in front to be used first Later expiry date in back Earlier expiry date in front “Use first” box for vaccines brought back unused from fixed or outreach sessions 8 | Rotavirus vaccine attributes and storage conditions, November 2020 Module 2 | 02

Handling Rota. Teq. TM with No VVM l In the absence of a VVM, it is impossible to know if – vaccines have been exposed to high temperatures (above 8 degrees) and – for how long and – how many times l It is extremely important that cold chain conditions are strictly maintained and closely monitored from vaccine receipt to vaccine administration. l Temperatures should be recorded twice daily on a monitoring chart and corrective action taken immediately if there is a cold chain failure. 9 | Rotavirus vaccine attributes and storage conditions, November 2020 Module 2 | 02

Handling Rota. Teq. TM with No VVM: Outreach (1/2) l Careful micro-planning is needed so that the appropriate numbers of Rotateq. TM tubes are placed in the vaccine carrier; this will minimize unnecessary wastage at the end of the session. l Discard unopened Rotateq. TM tubes at the end of outreach or mobile sessions if there is no assurance that the cold chain was maintained. 10 | Rotavirus vaccine attributes and storage conditions, November 2020 Module 2 | 02

Handling Rota. Teq. TM with No VVM: Outreach (2/2) l To avoid wastage, careful planning for outreach and mobile sessions is needed: – Keep a due list that has names of all infants needing Rotavirus vaccine (Rotateq. TM) – Put the Rota. Teq. TM vaccine in the vaccine carrier based on the number of infants expected to get the vaccine l Keep vaccine carrier in a cool place away from the sun l Keep the Rota. Teq. TM vaccine inside the vaccine carrier until you are ready to give the vaccine 11 | Rotavirus vaccine attributes and storage conditions, November 2020 Module 2 | 02

What should you do? The refrigerator stops functioning. What should you do? 12 | Rotavirus vaccine attributes and storage conditions, November 2020 Module 2 | 02

Key messages l Rotateq. TM is an oral vaccine in liquid formulation l Rotateq. TM comes in a single-dose squeezable 2 ml plastic tube l Irritability and loss of appetite are very common side effects of Rotateq. TM vaccine l Rotateq. TM vaccine comes without a VVM and therefore proper planning and handling is essential l Store vaccines between +2°C to +8°C l Vaccines with early expiration dates should be kept in front of the refrigerator to be used first l Do not open the refrigerator door often l Regularly monitor the temperature of the refrigerator 13 | Rotavirus vaccine attributes and storage conditions, November 2020 Module 2 | 02

End of module Thank you for your attention! 14 | Rotavirus vaccine attributes and storage conditions, November 2020 Module 2 | 02
 Rotavirus aikuisella
Rotavirus aikuisella Reovirus
Reovirus Carlo nnn
Carlo nnn Rotavirus
Rotavirus Rotavirus
Rotavirus Rotavirus
Rotavirus Rotavirus
Rotavirus C device module module 1
C device module module 1 Fspos vägledning för kontinuitetshantering
Fspos vägledning för kontinuitetshantering Typiska novell drag
Typiska novell drag Nationell inriktning för artificiell intelligens
Nationell inriktning för artificiell intelligens Returpilarna
Returpilarna Varför kallas perioden 1918-1939 för mellankrigstiden
Varför kallas perioden 1918-1939 för mellankrigstiden En lathund för arbete med kontinuitetshantering
En lathund för arbete med kontinuitetshantering Särskild löneskatt för pensionskostnader
Särskild löneskatt för pensionskostnader