European Medicines Verification Organisation EMVO What is EMVO









- Slides: 33

European Medicines Verification Organisation (EMVO) What is EMVO and how does EU Hub function? Tobias Beer (Head of Commercial & Partner Management) www. emvo-medicines. eu helpdesk@emvo-medicines. eu dd/mm/yyyy

Agenda European Medicines Verification System How to get connected? Technical On-boarding Master Data-flow and Multi-country packs 31/03/2017 e-verifisering av legemiddelpakninger 2

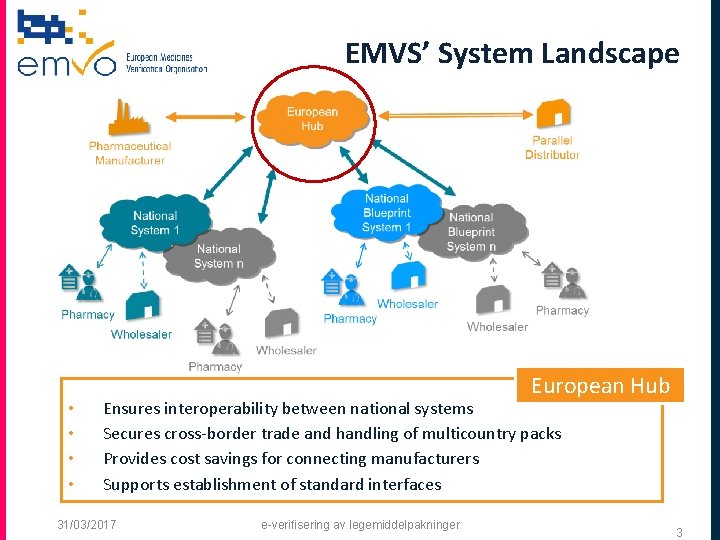
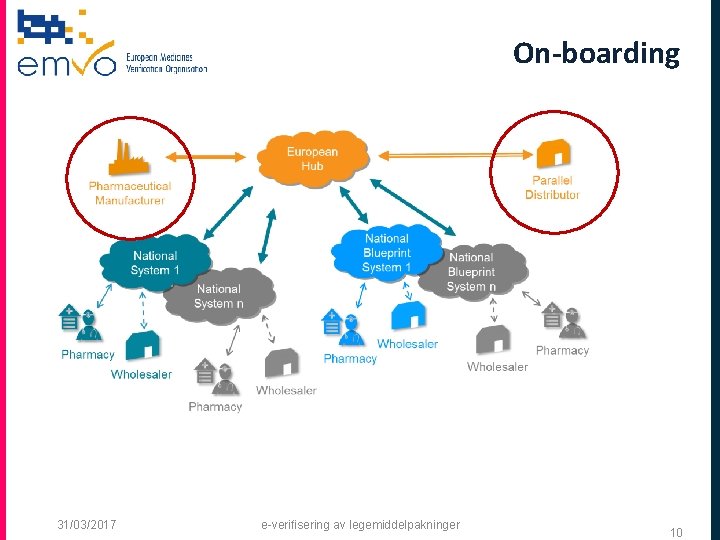
EMVS’ System Landscape • • European Hub Ensures interoperability between national systems Secures cross-border trade and handling of multicountry packs Provides cost savings for connecting manufacturers Supports establishment of standard interfaces 31/03/2017 e-verifisering av legemiddelpakninger 3

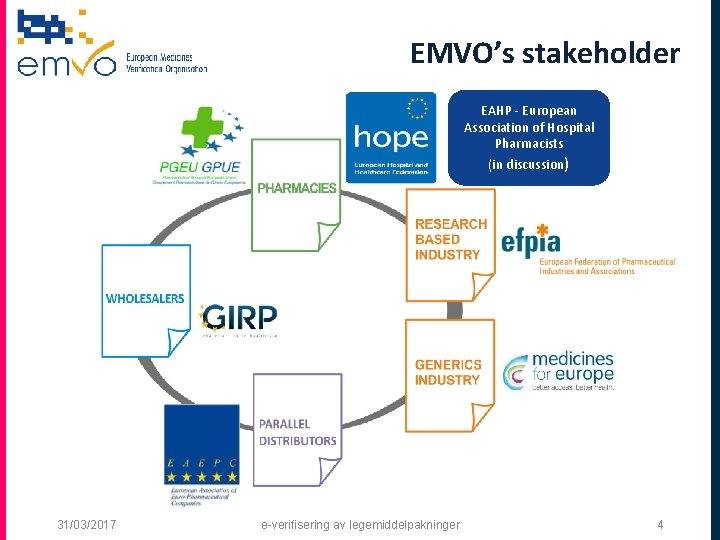
EMVO’s stakeholder EAHP - European Association of Hospital Pharmacists (in discussion) 31/03/2017 e-verifisering av legemiddelpakninger 4

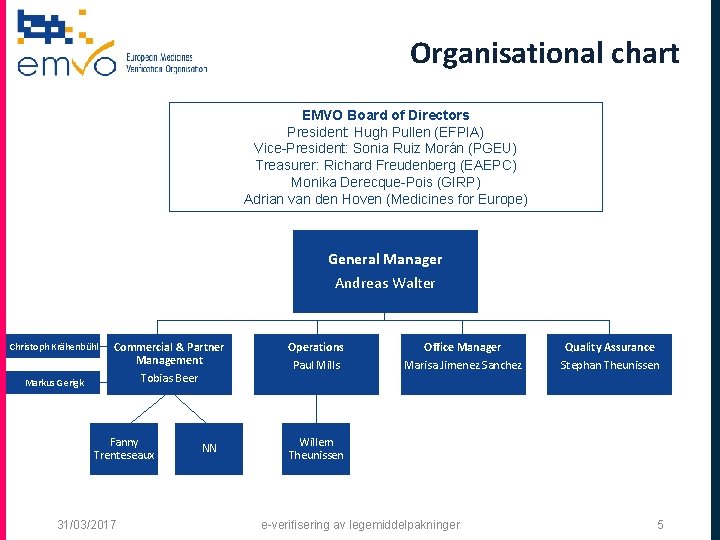
Organisational chart EMVO Board of Directors President: Hugh Pullen (EFPIA) Vice-President: Sonia Ruiz Morán (PGEU) Treasurer: Richard Freudenberg (EAEPC) Monika Derecque-Pois (GIRP) Adrian van den Hoven (Medicines for Europe) General Manager Andreas Walter Christoph Krähenbühl Commercial & Partner Management Tobias Beer Markus Gerigk Fanny Trenteseaux 31/03/2017 NN Operations Paul Mills Office Manager Marisa Jimenez Sanchez Quality Assurance Stephan Theunissen Willem Theunissen e-verifisering av legemiddelpakninger 5

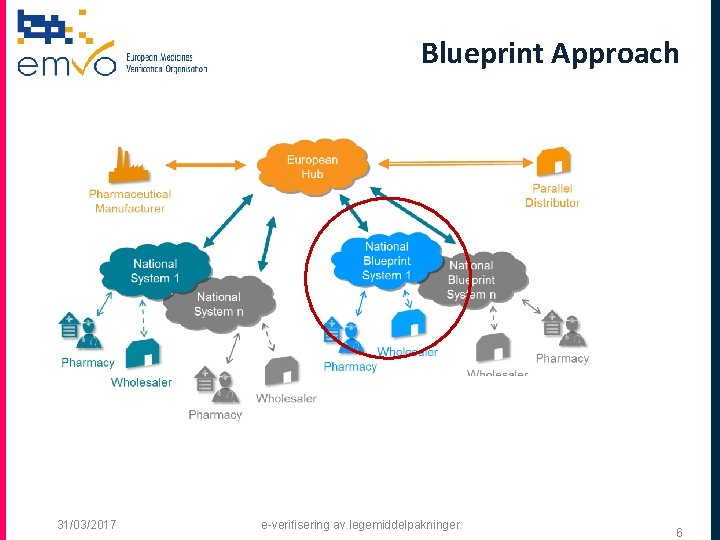
Blueprint Approach 31/03/2017 e-verifisering av legemiddelpakninger 6

Elements of Blueprint Approach q Full Stakeholder participation in NMVO Governance q Implementation of national systems based on compliance with EMVO URS q Technical operation by an IT Blueprint Provider q Support by EMVO during deployment process 31/03/2017 e-verifisering av legemiddelpakninger 7

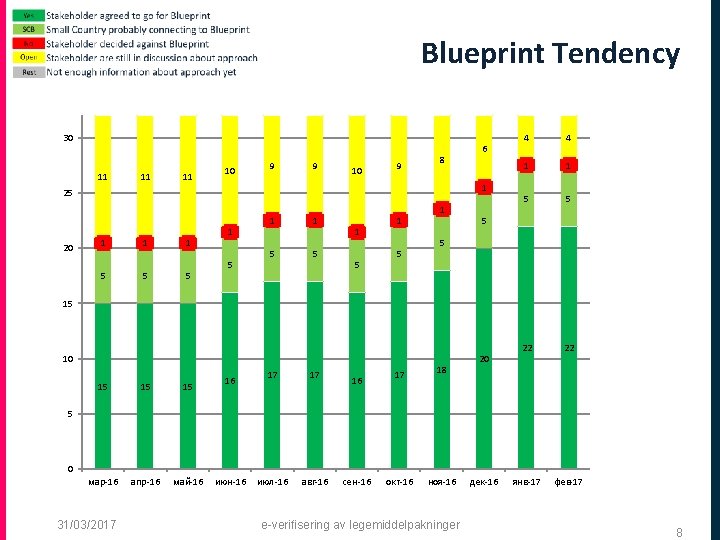
Blueprint Tendency 30 11 11 11 10 9 9 10 9 8 1 25 20 1 1 1 5 5 5 6 1 5 1 1 5 5 1 5 1 4 4 1 1 5 5 22 22 янв-17 фев-17 5 5 15 10 15 15 15 мар-16 апр-16 май-16 16 17 17 июл-16 авг-16 16 17 18 20 5 0 31/03/2017 июн-16 сен-16 окт-16 ноя-16 e-verifisering av legemiddelpakninger дек-16 8

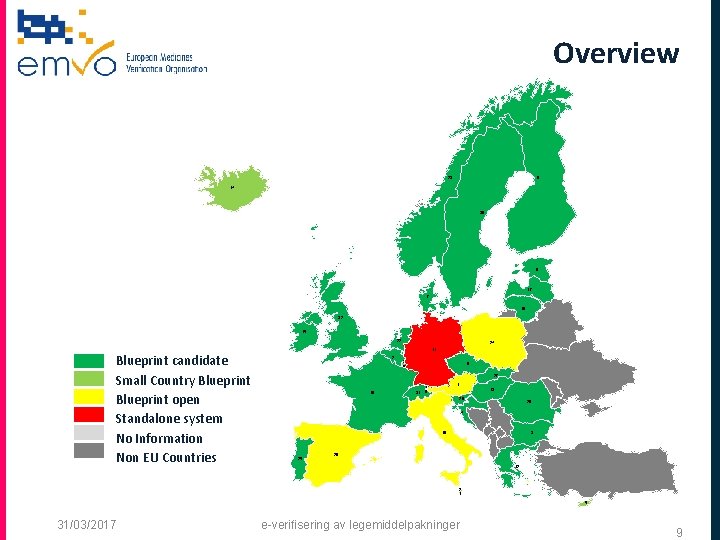
Overview 9 23 14 30 8 17 7 19 32 15 22 Blueprint candidate Small Country Blueprint open Standalone system No Information Non EU Countries 24 11 2 2 0 6 27 10 31 1 1 8 13 28 26 4 3 16 25 29 12 2 1 5 31/03/2017 e-verifisering av legemiddelpakninger 9

On-boarding 31/03/2017 e-verifisering av legemiddelpakninger 10

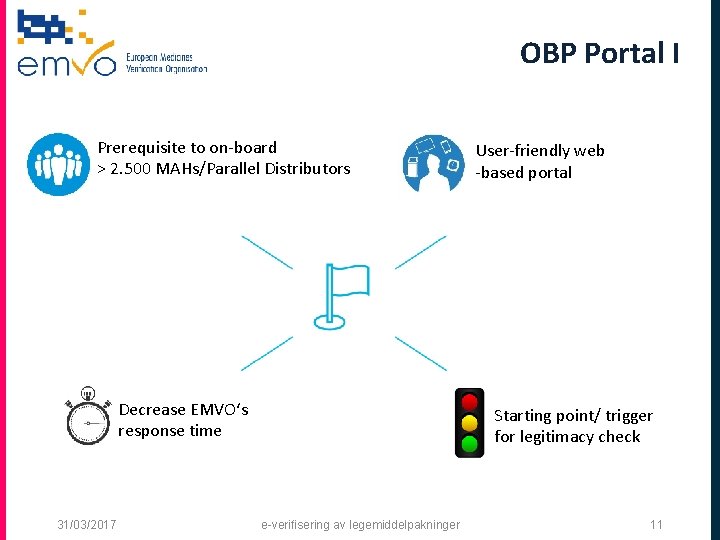
OBP Portal I Prerequisite to on-board > 2. 500 MAHs/Parallel Distributors Decrease EMVO‘s response time 31/03/2017 User-friendly web -based portal Starting point/ trigger for legitimacy check e-verifisering av legemiddelpakninger 11

OBP Portal II https: //www. emvo-medicines. eu/eu-hub-on-boarding/obp-portal/ 31/03/2017 e-verifisering av legemiddelpakninger 12

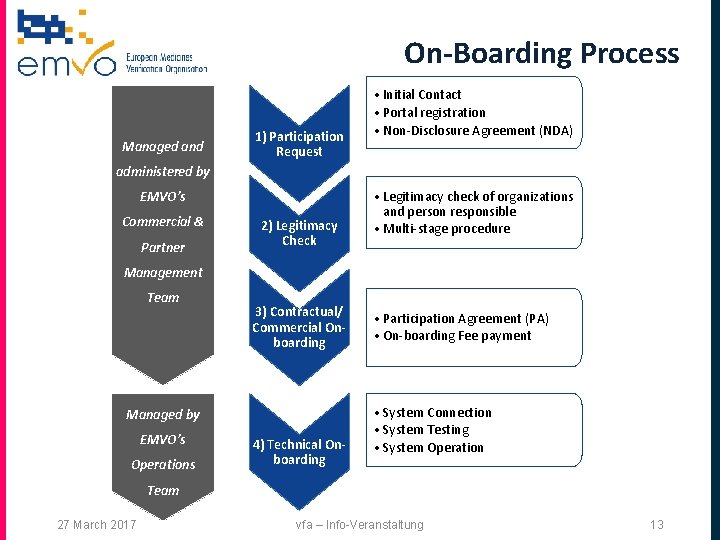
On-Boarding Process Managed and 1) Participation Request • Initial Contact • Portal registration • Non-Disclosure Agreement (NDA) administered by EMVO’s Commercial & Partner 2) Legitimacy Check • Legitimacy check of organizations and person responsible • Multi-stage procedure Management Team 3) Contractual/ Commercial Onboarding Managed by EMVO’s Operations 4) Technical Onboarding • Participation Agreement (PA) • On-boarding Fee payment • System Connection • System Testing • System Operation Team 27 March 2017 vfa – Info-Veranstaltung 13

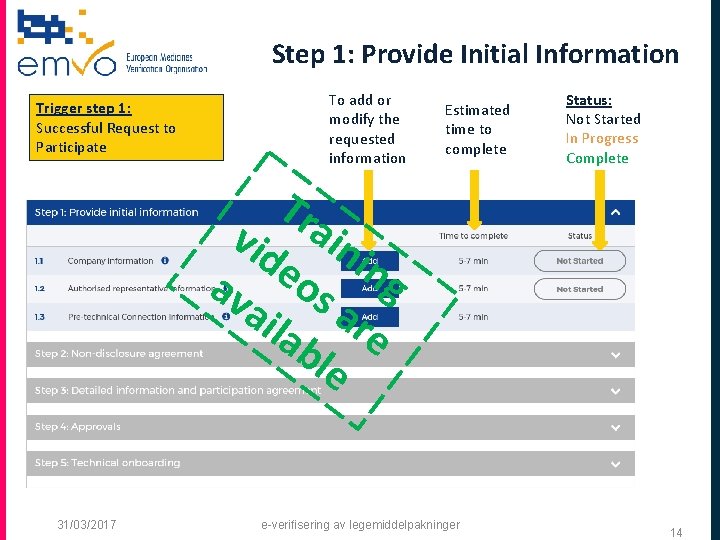
Step 1: Provide Initial Information Trigger step 1: Successful Request to Participate To add or modify the requested information Estimated time to complete Status: Not Started In Progress Complete Tr vid ain i n e o g av s ail ar ab e le 31/03/2017 e-verifisering av legemiddelpakninger 14

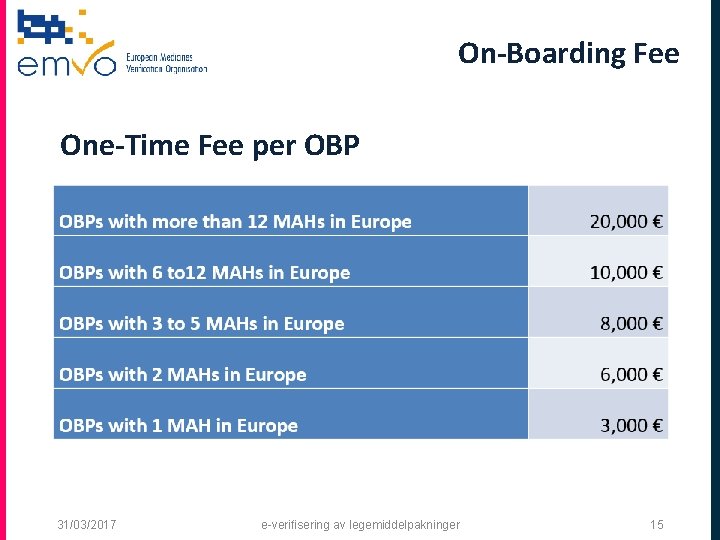
On-Boarding Fee One-Time Fee per OBP 31/03/2017 e-verifisering av legemiddelpakninger 15

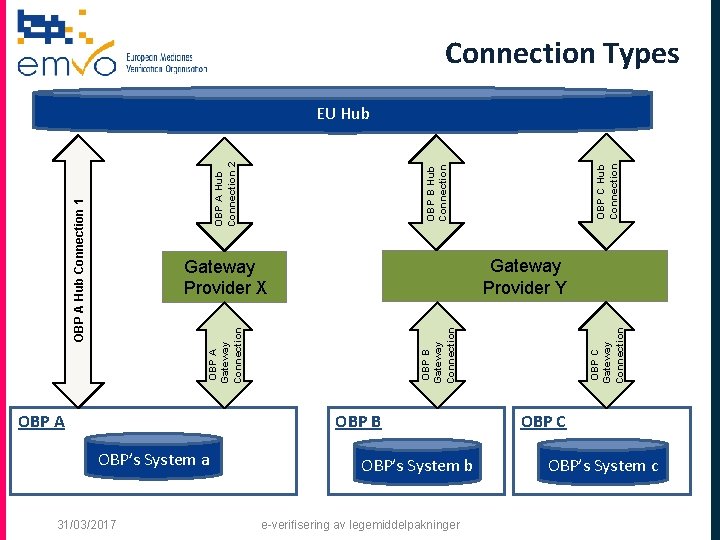
Technical On-boarding q Starts when Participation Agreement is signed by EMVO q OBP will connect his client sequentially to: § the ITE environment to develop an interface § the IQE environment to certify his interface § The PRD environment to use his interface q OBP can choose to connect via § a Direct connection (data send from client straight to EU Hub) § a Gateway connection (Data sent first to a Gateway Provider. The Gateway Provider sends it through to the EU Hub

Connection Types Gateway Provider Y OBP B OBP’s System a 31/03/2017 OBP’s System b e-verifisering av legemiddelpakninger OBP C Gateway Connection OBP B Gateway Connection OBP A Gateway Connection Gateway Provider X OBP A OBP C Hub Connection OBP B Hub Connection OBP A Hub Connection 1 OBP A Hub Connection 2 EU Hub OBP C OBP’s System c

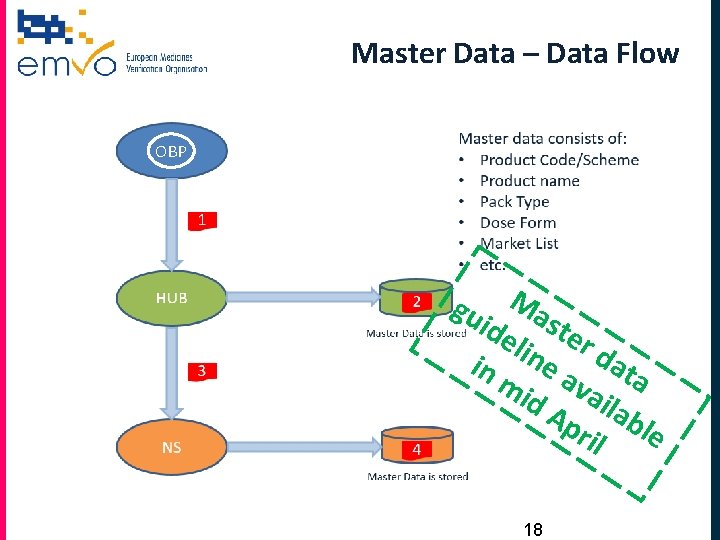
Master Data – Data Flow OBP gu Mas ide te lin r d in e a ata mi va d A ila pri ble l 18

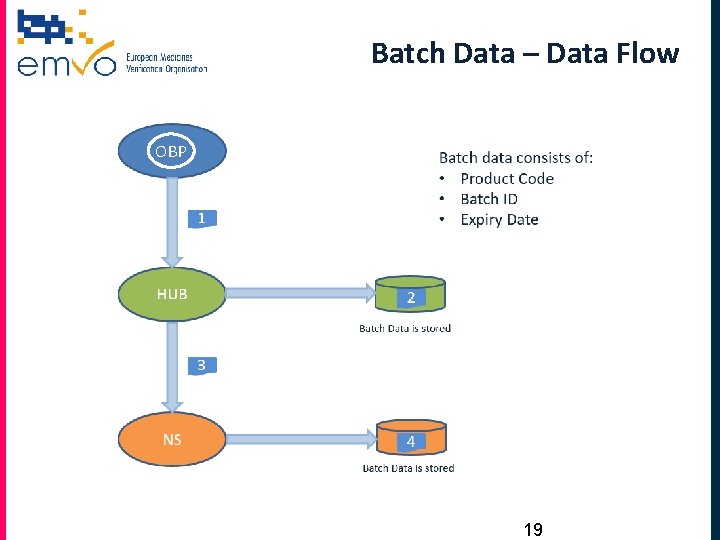
Batch Data – Data Flow OBP 19

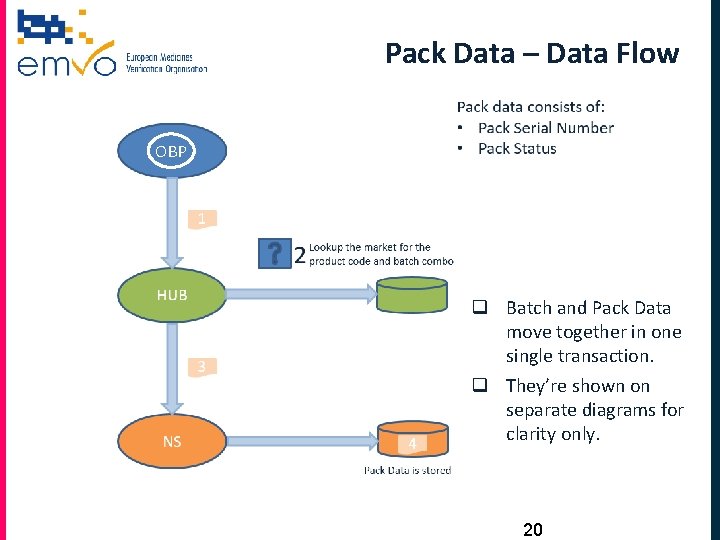
Pack Data – Data Flow OBP q Batch and Pack Data move together in one single transaction. q They’re shown on separate diagrams for clarity only. 20

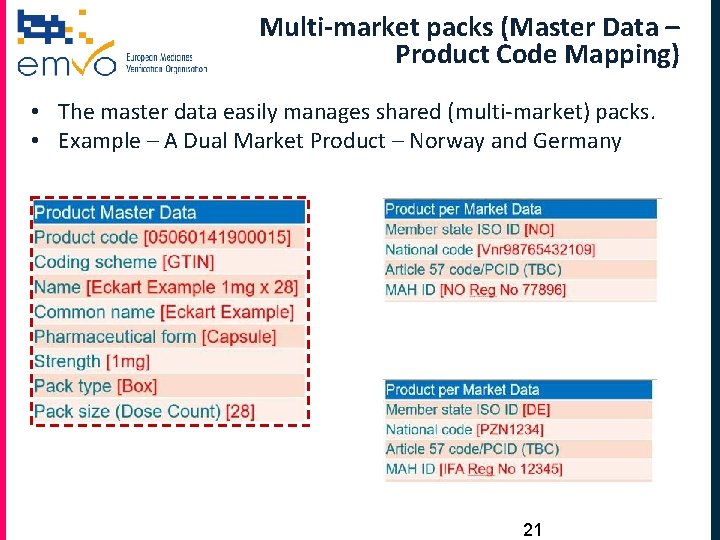
Multi-market packs (Master Data – Product Code Mapping) • The master data easily manages shared (multi-market) packs. • Example – A Dual Market Product – Norway and Germany 21

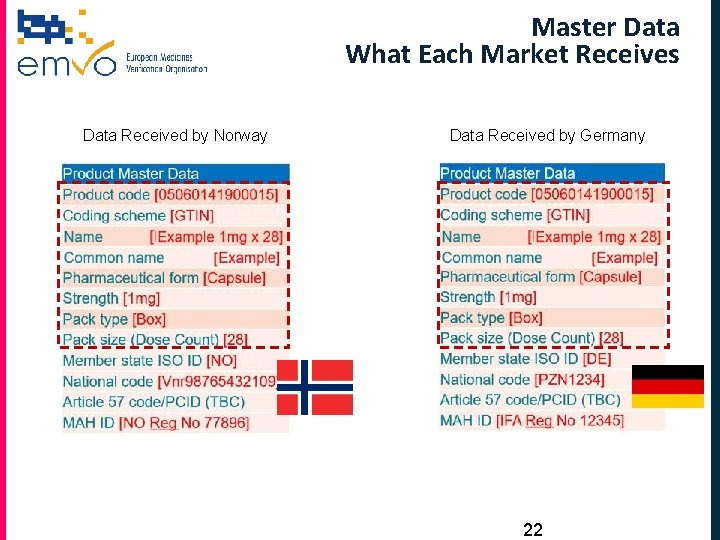
Master Data What Each Market Receives Data Received by Norway Data Received by Germany 22

Look-up process • Client (Pharmacy/Wholesaler) Application reads: – 0109504000059118107654321 D<GS>171411202110987654 d 32 – Database gives 09504000059118 ≡ Vnr “ 1312345678913” 23

Contact details NMVOs 31/03/2017 e-verifisering av legemiddelpakninger 24

Contact details NMVOs 31/03/2017 e-verifisering av legemiddelpakninger 25

31/03/2017 e-verifisering av legemiddelpakninger 26

THANK YOU for your ATTENTION! 31/03/2017 e-verifisering av legemiddelpakninger 27

31/03/2017 e-verifisering av legemiddelpakninger 28

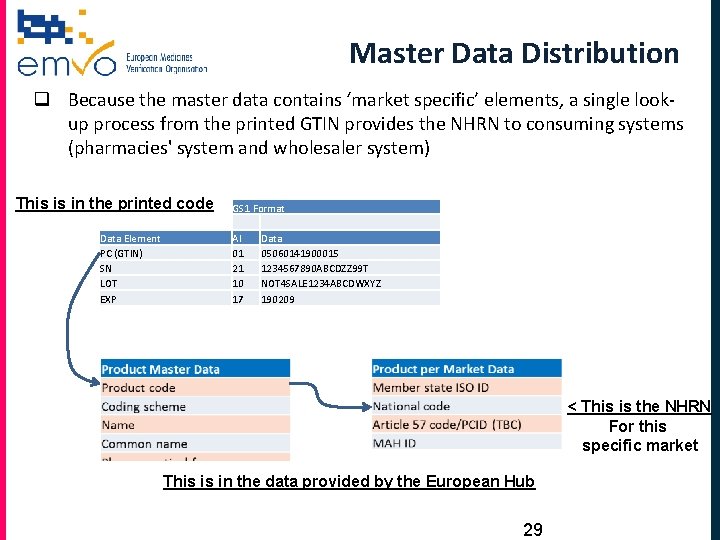
Master Data Distribution q Because the master data contains ‘market specific’ elements, a single lookup process from the printed GTIN provides the NHRN to consuming systems (pharmacies' system and wholesaler system) This is in the printed code Data Element PC (GTIN) SN LOT EXP GS 1 Format AI 01 21 10 17 Data 05060141900015 1234567890 ABCDZZ 99 T NOT 4 SALE 1234 ABCDWXYZ 190209 < This is the NHRN For this specific market This is in the data provided by the European Hub 29

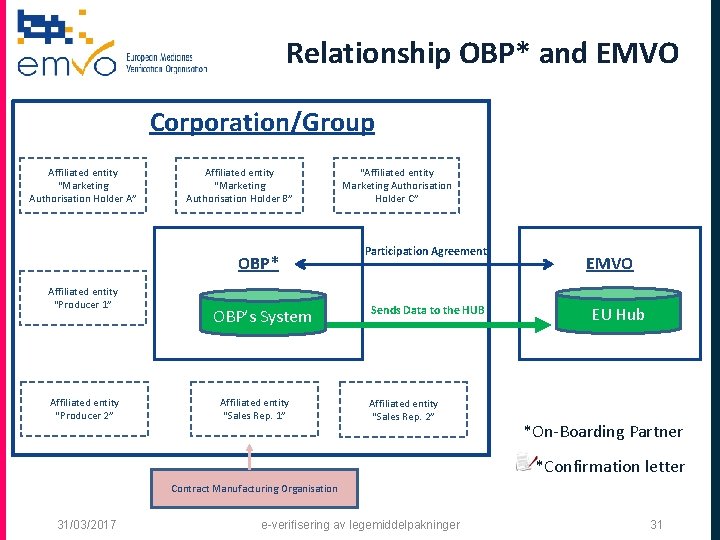
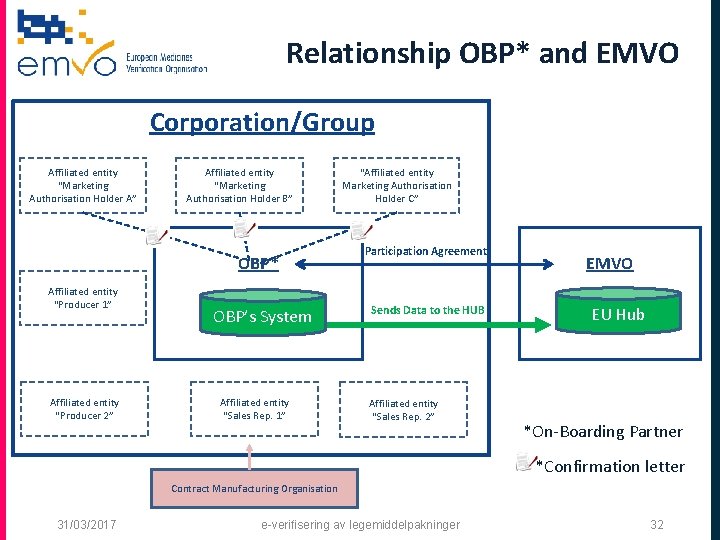
What is an “OBP”? q OBP means On-Boarding Partner and is the contracting party of EMVO and concludes the non-disclosure agreement (NDA) and participation agreement (PA). q The OBP is legally authorized to sign on behalf of a MAH/a group of MAHs. q The OBP has to be affiliated (*) to a MAH/a group of MAHs (*) Affiliate shall mean, in relation to a Party, any other person affiliated with such Party within the meaning of Article 11 of the Belgian Code of Companies (it being understood, for the avoidance of doubt, that the definition set out in said Article 11 is agreed to also apply to non-Belgian persons). 31/03/2017 e-verifisering av legemiddelpakninger 30

Relationship OBP* and EMVO Corporation/Group Affiliated entity “Marketing Authorisation Holder A” Affiliated entity “Marketing Authorisation Holder B” OBP* Affiliated entity “Producer 1” Affiliated entity “Producer 2” OBP’s System Affiliated entity “Sales Rep. 1” “Affiliated entity Marketing Authorisation Holder C” Participation Agreement Sends Data to the HUB Affiliated entity “Sales Rep. 2” EMVO EU Hub *On-Boarding Partner *Confirmation letter Contract Manufacturing Organisation 31/03/2017 e-verifisering av legemiddelpakninger 31

Relationship OBP* and EMVO Corporation/Group Affiliated entity “Marketing Authorisation Holder A” Affiliated entity “Marketing Authorisation Holder B” OBP* Affiliated entity “Producer 1” Affiliated entity “Producer 2” OBP’s System Affiliated entity “Sales Rep. 1” “Affiliated entity Marketing Authorisation Holder C” Participation Agreement Sends Data to the HUB Affiliated entity “Sales Rep. 2” EMVO EU Hub *On-Boarding Partner *Confirmation letter Contract Manufacturing Organisation 31/03/2017 e-verifisering av legemiddelpakninger 32

DISCLAIMER 31/03/2017 e-verifisering av legemiddelpakninger 33
 Abasagar
Abasagar European directorate for the quality of medicines
European directorate for the quality of medicines Emvo helpdesk
Emvo helpdesk Glencoe health chapter 19 medicines and drugs
Glencoe health chapter 19 medicines and drugs National medicines policy
National medicines policy Tsaang gubat benefit
Tsaang gubat benefit Ectoparasiticides veterinary medicines
Ectoparasiticides veterinary medicines Medicines information centre
Medicines information centre Staff of marvelous medicines
Staff of marvelous medicines Ggc medicines
Ggc medicines Summarize roosevelt's approach to environmental problems
Summarize roosevelt's approach to environmental problems Medicines complete
Medicines complete Medicines complete martindale
Medicines complete martindale Refrigerant management program
Refrigerant management program Federal agency for medicines and health products
Federal agency for medicines and health products Nhs dictionary of medicines and devices
Nhs dictionary of medicines and devices Veterinary medicines directorate
Veterinary medicines directorate Cqc medicines management
Cqc medicines management Pharmaceutical schedule
Pharmaceutical schedule Which type of drugs are distributed by envelope method
Which type of drugs are distributed by envelope method Medicines learning portal
Medicines learning portal Verification meaning
Verification meaning Analog mixed signal verification
Analog mixed signal verification Nws verification
Nws verification Cug card
Cug card Behavioral variability aba
Behavioral variability aba Cdsl edis
Cdsl edis Chapter 2 pharmacy law ethics and regulatory agencies
Chapter 2 pharmacy law ethics and regulatory agencies Nsi verification form
Nsi verification form Tncompass
Tncompass Dea calculation
Dea calculation Balance calibration sop
Balance calibration sop Asset verification software
Asset verification software Withdrawal design
Withdrawal design