Estimating Avogadros Number Chemistry 141 Lab Handout Stearic

Estimating Avogadro’s Number Chemistry 141 Lab Handout
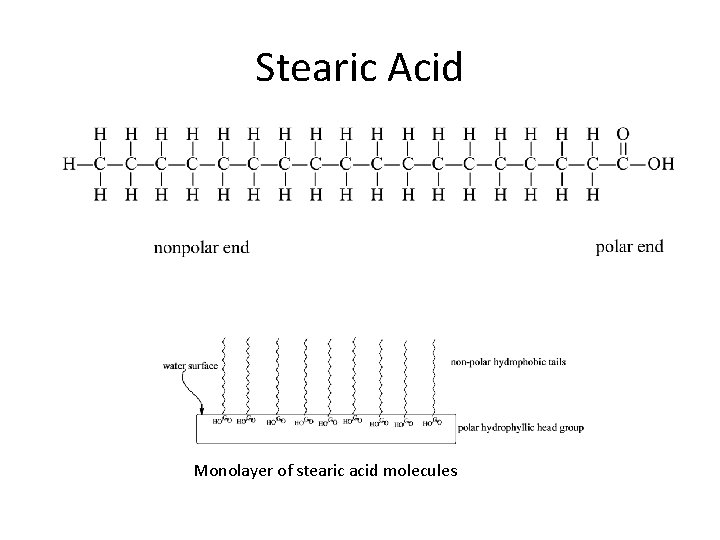
Stearic Acid Monolayer of stearic acid molecules
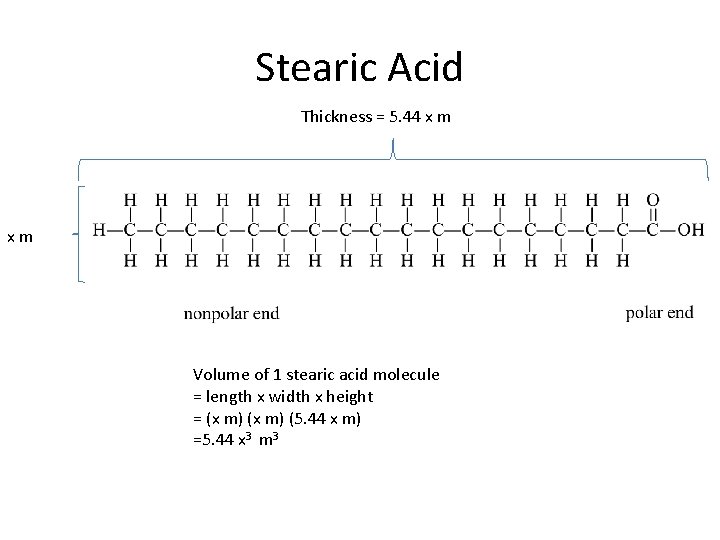
Stearic Acid Thickness = 5. 44 x m xm Volume of 1 stearic acid molecule = length x width x height = (x m) (5. 44 x m) =5. 44 x 3 m 3

Part 1: Calibration of the pipet. • Wash and dry a 10 m. L beaker. – – – 3 x ammonia wash 3 x deionized water rinse 1 x acetone rinse Dry well with paper towel 3 x hexane rinse (1/2 m. L per rinse) ALL WASTE HEXANE MUST BE DISPOSED OF IN THE WASTE CONTAINERS. DO NOT DUMP HEXANE IN THE SINK. • Fill 10 m. L beaker with hexane. • Transfer hexane drop-wise to a clean dry 10 -m. L graduated cylinder to reach a volume of 1 ml. Record the number of drops. • Repeat until 2 trials are within 10% of each other. • Dispose of excess hexane in the appropriate waste container. • Calculate average volume per drop of hexane.
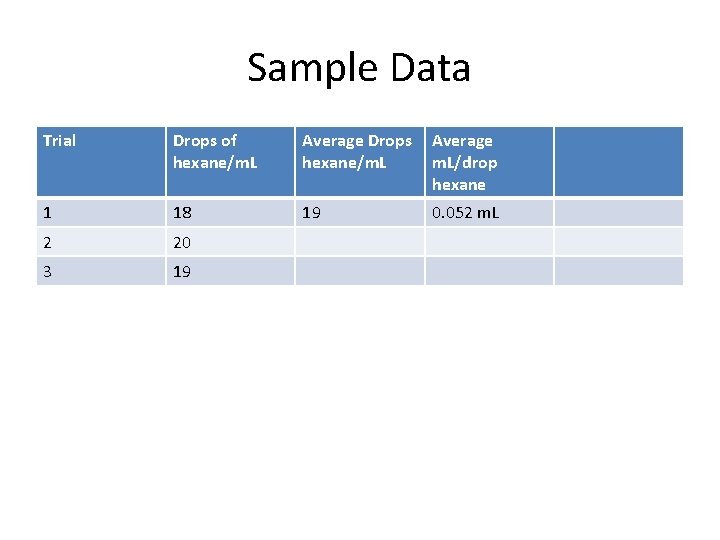
Sample Data Trial Drops of hexane/m. L Average Drops hexane/m. L Average m. L/drop hexane 1 18 19 0. 052 m. L 2 20 3 19

Part II: The Monolayer. • Clean a watchglass with soap and water, ammonia, and deionized water. Hold the glass by the edges to avoid putting fingerprints on it. • Place the watch glass on a ring and make sure that the watch glass is parallel to the floor. Fill the watch glass to the brim with deionized water. • Fill clean 10 m. L beaker with stearic acid/hexane solution from under the hood. • Add stearic acid/hexane dropwise to the watchglass. Count the drops. – The solution should spread out rapidly across the surface of the water and disappear within a few seconds. If the watch glass is not properly cleaned then an oily residue may appear after only a few drops of solution. In this case it will be necessary to clean the watch glass again. If the first few drops disappear rapidly, continue adding the solution drop-wise, counting the drops. After approximately 20 drops, as the monolayer nears completion, the drop of solution forms a circular pattern rather than flowering out. The circular film of solution contracts as it evaporates and disappears in a relatively short time. This pattern will be observed for a few drops until finally, one drop strikes the surface and remains as a lens or globule that requires a prolonged period of time to disappear. Record the number of drops. At this point, the surface of the water is covered with a monolayer of stearic acid and one more drop placed at a different point on the water surface forms a second “lens”.
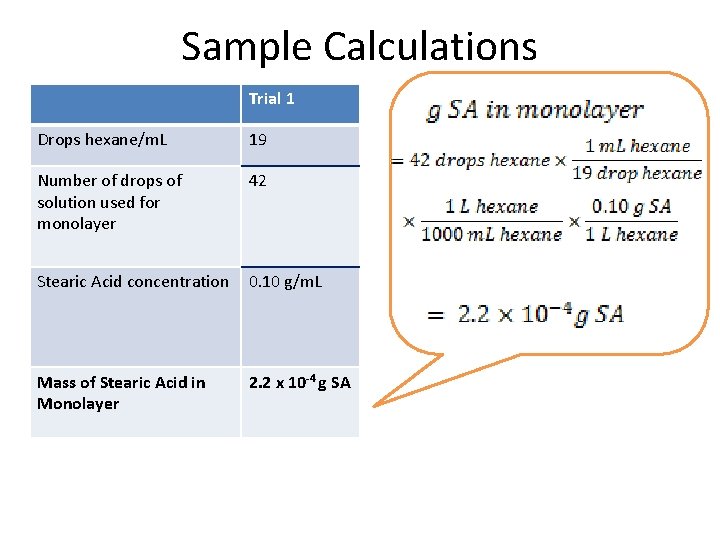
Sample Calculations Trial 1 Drops hexane/m. L 19 Number of drops of solution used for monolayer 42 Stearic Acid concentration 0. 10 g/m. L Mass of Stearic Acid in Monolayer 2. 2 x 10 -4 g SA
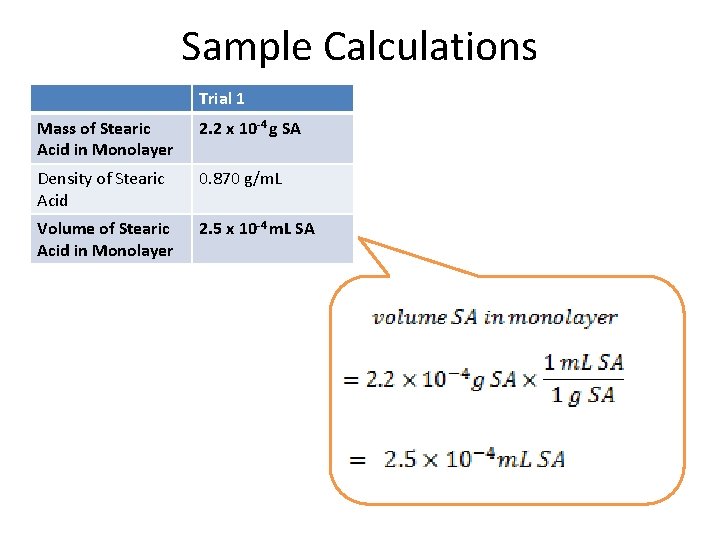
Sample Calculations Trial 1 Mass of Stearic Acid in Monolayer 2. 2 x 10 -4 g SA Density of Stearic Acid 0. 870 g/m. L Volume of Stearic Acid in Monolayer 2. 5 x 10 -4 m. L SA
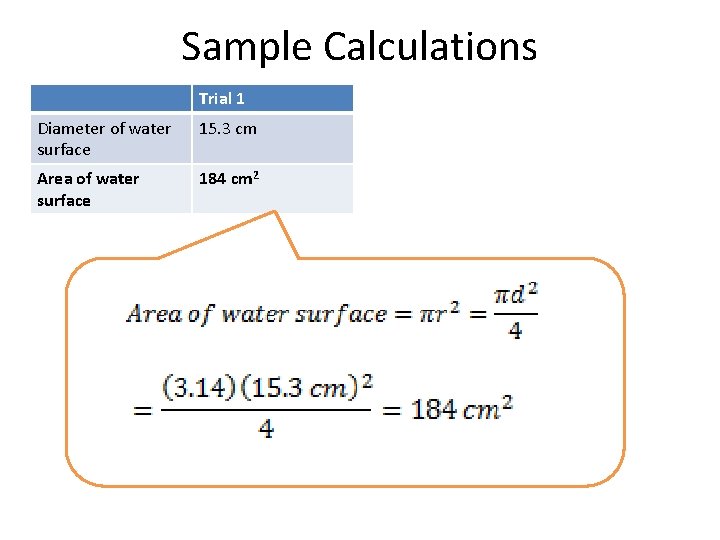
Sample Calculations Trial 1 Diameter of water surface 15. 3 cm Area of water surface 184 cm 2
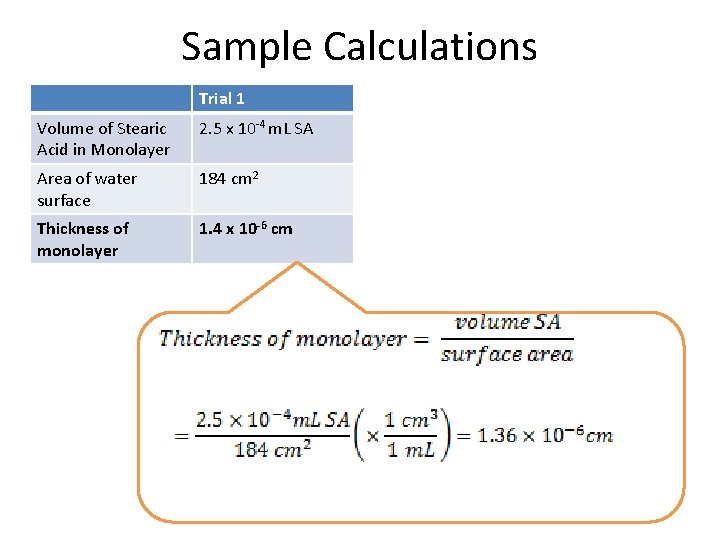
Sample Calculations Trial 1 Volume of Stearic Acid in Monolayer 2. 5 x 10 -4 m. L SA Area of water surface 184 cm 2 Thickness of monolayer 1. 4 x 10 -6 cm
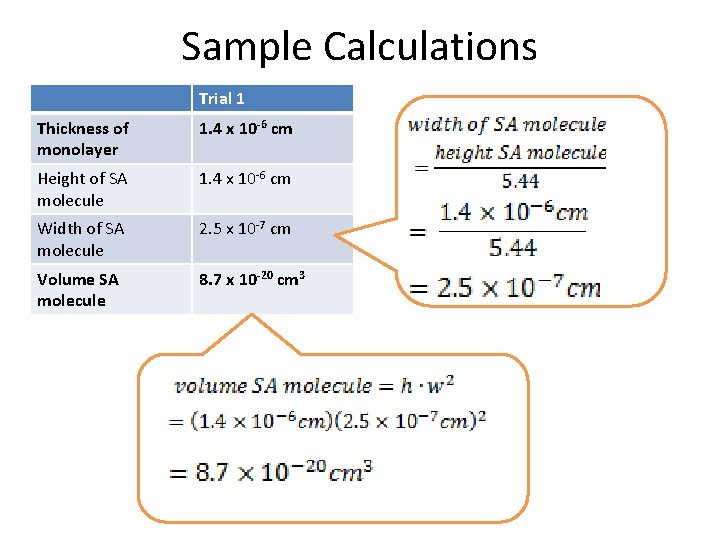
Sample Calculations Trial 1 Thickness of monolayer 1. 4 x 10 -6 cm Height of SA molecule 1. 4 x 10 -6 cm Width of SA molecule 2. 5 x 10 -7 cm Volume SA molecule 8. 7 x 10 -20 cm 3
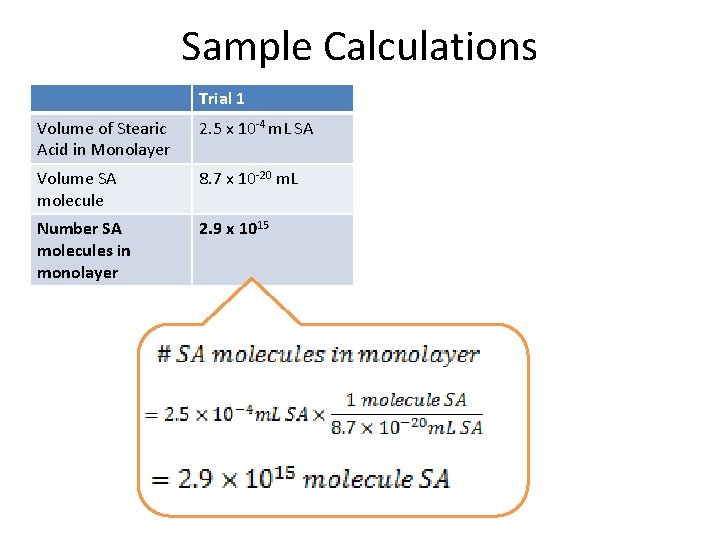
Sample Calculations Trial 1 Volume of Stearic Acid in Monolayer 2. 5 x 10 -4 m. L SA Volume SA molecule 8. 7 x 10 -20 m. L Number SA molecules in monolayer 2. 9 x 1015
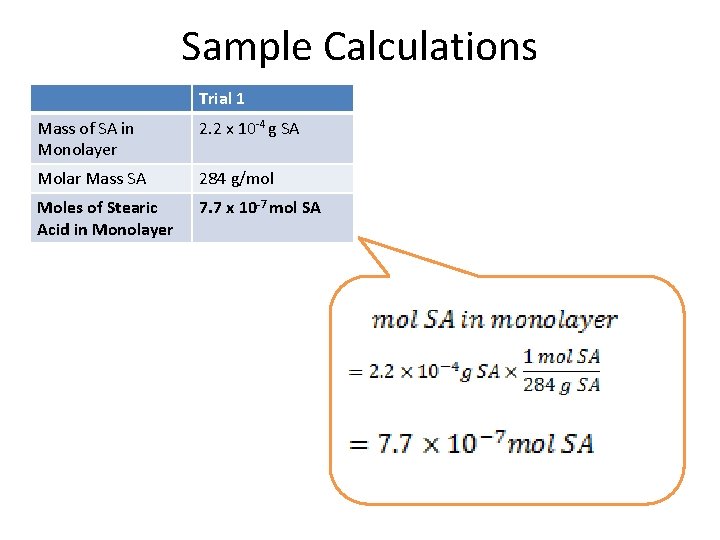
Sample Calculations Trial 1 Mass of SA in Monolayer 2. 2 x 10 -4 g SA Molar Mass SA 284 g/mol Moles of Stearic Acid in Monolayer 7. 7 x 10 -7 mol SA
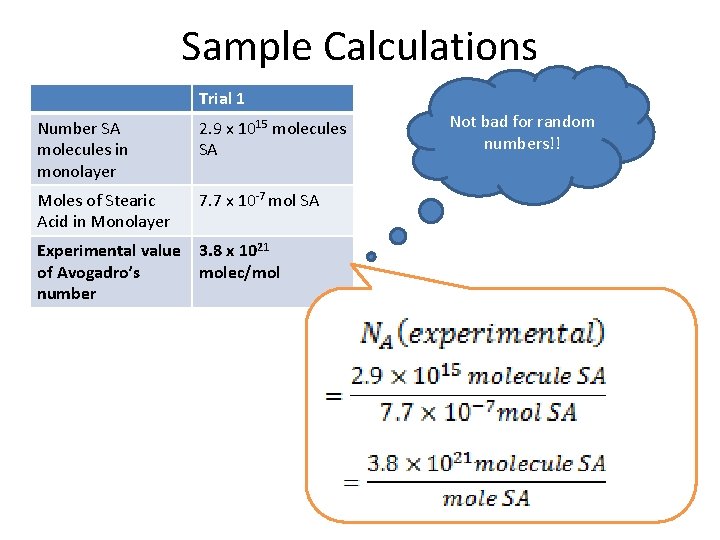
Sample Calculations Trial 1 Number SA molecules in monolayer 2. 9 x 1015 molecules SA Moles of Stearic Acid in Monolayer 7. 7 x 10 -7 mol SA Experimental value of Avogadro’s number 3. 8 x 1021 molec/mol Not bad for random numbers!!
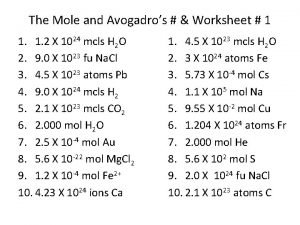
- Slides: 14