Enthalpy and Hesss Law Chemistry I Chemical Reactions




- Slides: 13

Enthalpy and Hess’s Law Chemistry I

Chemical Reactions at Constant Volume and Pressure n We know DE = q + w ¨ DE = q – PDV ¨ At constant volume, PDV = 0 ¨ So at constant volume, DE = qv ¨ Subscript “v” reminds us that is at constant volume

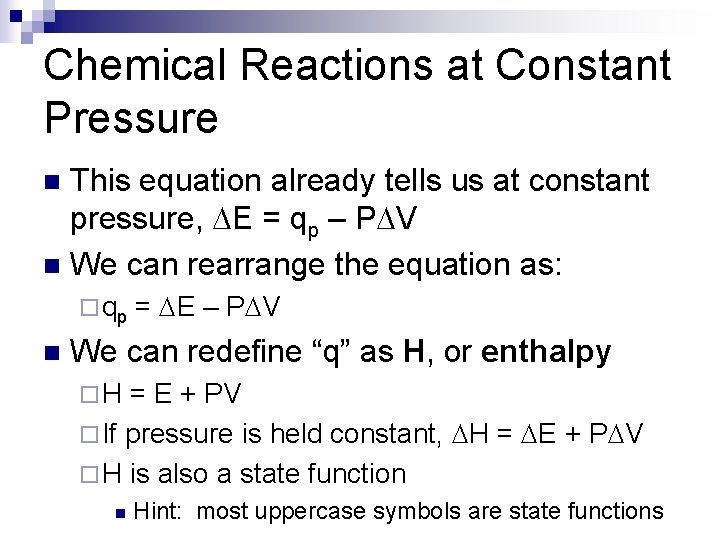
Chemical Reactions at Constant Pressure This equation already tells us at constant pressure, DE = qp – PDV n We can rearrange the equation as: n ¨ qp n = DE – PDV We can redefine “q” as H, or enthalpy ¨H = E + PV ¨ If pressure is held constant, DH = DE + PDV ¨ H is also a state function n Hint: most uppercase symbols are state functions

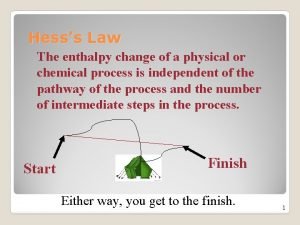
Enthalpy n Enthalpy – heat absorbed or released in a reaction ¨ Depends on the difference in quantity of heat ¨ Represented by a H n When the pressure of a reaction remains constant, the heat absorbed or released during a chemical reaction is equal to the change in enthalpy for the reaction

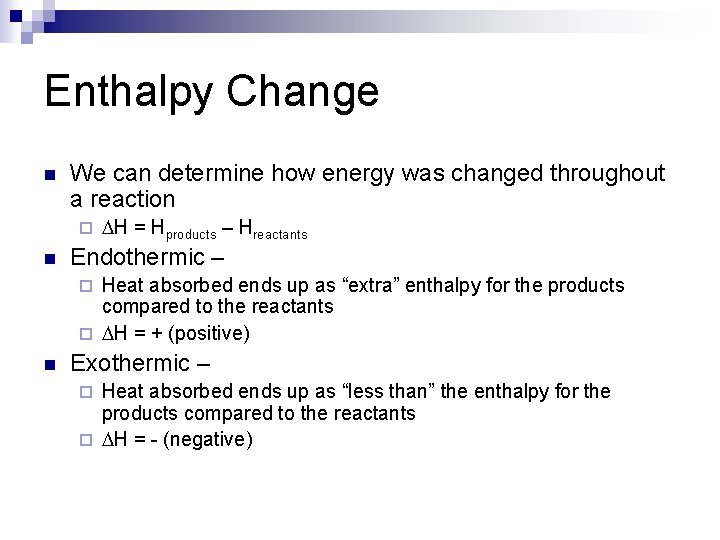
Enthalpy Change n We can determine how energy was changed throughout a reaction ¨ n DH = Hproducts – Hreactants Endothermic – Heat absorbed ends up as “extra” enthalpy for the products compared to the reactants ¨ DH = + (positive) ¨ n Exothermic – Heat absorbed ends up as “less than” the enthalpy for the products compared to the reactants ¨ DH = - (negative) ¨

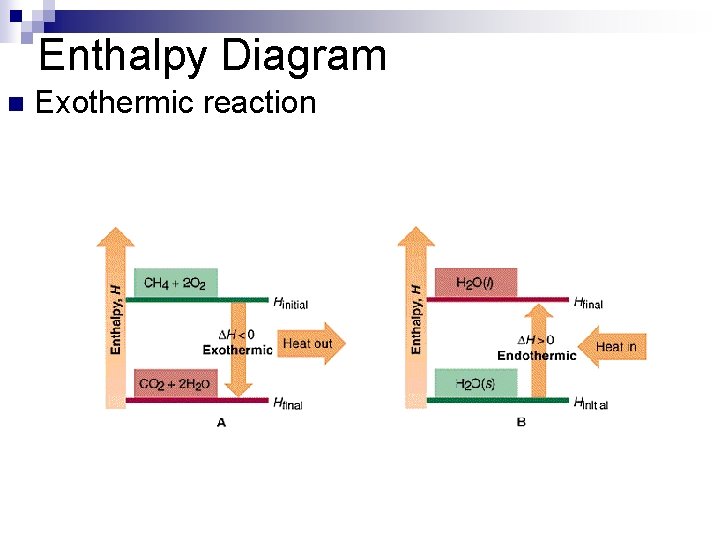
Enthalpy Diagram n Exothermic reaction

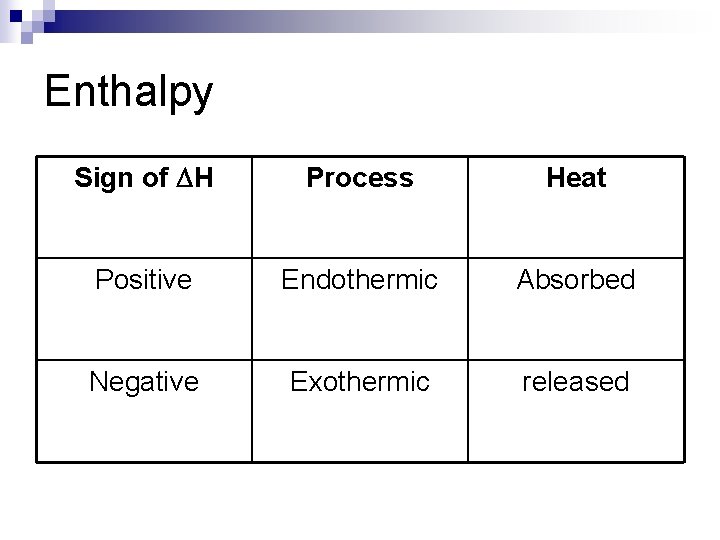
Enthalpy Sign of DH Process Heat Positive Endothermic Absorbed Negative Exothermic released

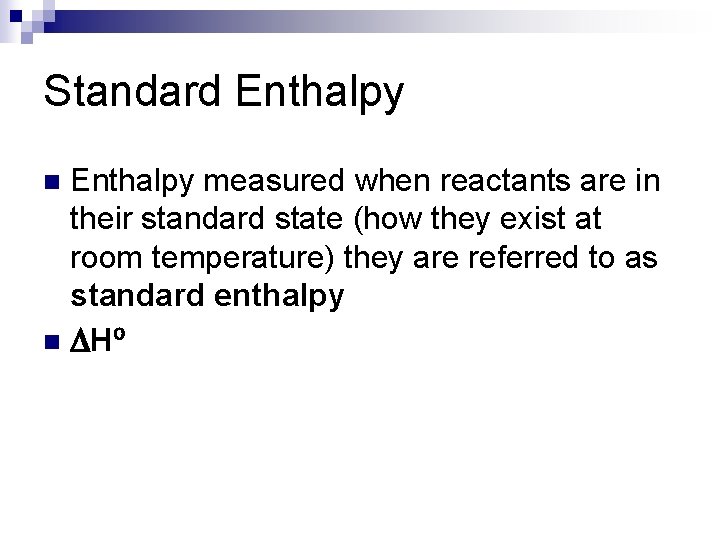
Standard Enthalpy measured when reactants are in their standard state (how they exist at room temperature) they are referred to as standard enthalpy n DH⁰ n

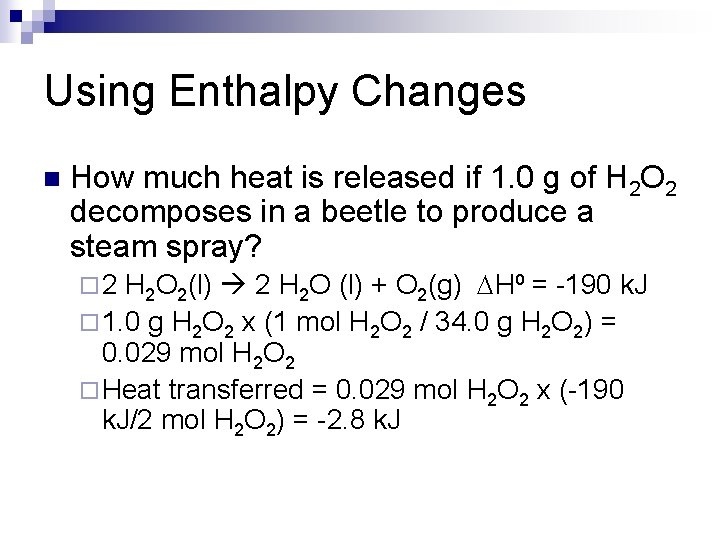
Using Enthalpy Changes n How much heat is released if 1. 0 g of H 2 O 2 decomposes in a beetle to produce a steam spray? H 2 O 2(l) 2 H 2 O (l) + O 2(g) DH⁰ = -190 k. J ¨ 1. 0 g H 2 O 2 x (1 mol H 2 O 2 / 34. 0 g H 2 O 2) = 0. 029 mol H 2 O 2 ¨ Heat transferred = 0. 029 mol H 2 O 2 x (-190 k. J/2 mol H 2 O 2) = -2. 8 k. J ¨ 2

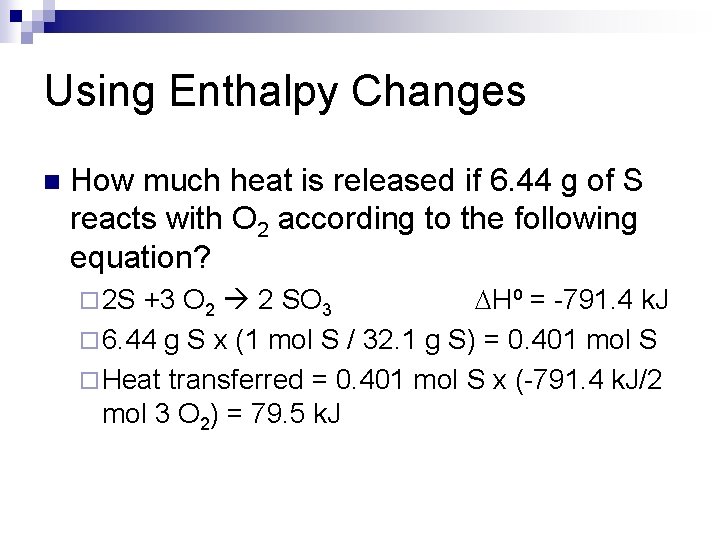
Using Enthalpy Changes n How much heat is released if 6. 44 g of S reacts with O 2 according to the following equation? +3 O 2 2 SO 3 DH⁰ = -791. 4 k. J ¨ 6. 44 g S x (1 mol S / 32. 1 g S) = 0. 401 mol S ¨ Heat transferred = 0. 401 mol S x (-791. 4 k. J/2 mol 3 O 2) = 79. 5 k. J ¨ 2 S

Relationship between DH and DE Under constant volume, DE =qv n Under constant pressure, DH = qp n For most reactions not involving gases, DE is almost the same as DH since there (since there is no change in DV) n For gases, some of the energy (DE) is used to expand (or contract) the gas, therefore DH will be slightly smaller (DV is changing) n

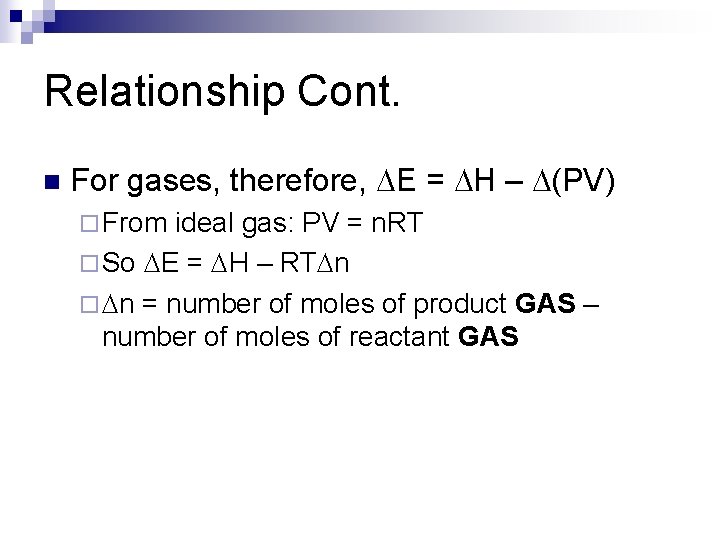
Relationship Cont. n For gases, therefore, DE = DH – D(PV) ¨ From ideal gas: PV = n. RT ¨ So DE = DH – RTDn ¨ Dn = number of moles of product GAS – number of moles of reactant GAS

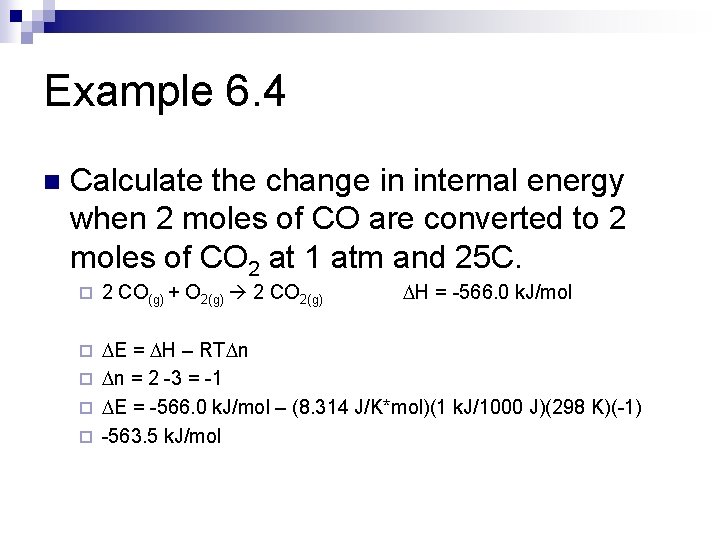
Example 6. 4 n Calculate the change in internal energy when 2 moles of CO are converted to 2 moles of CO 2 at 1 atm and 25 C. ¨ 2 CO(g) + O 2(g) 2 CO 2(g) DH = -566. 0 k. J/mol DE = DH – RTDn ¨ Dn = 2 -3 = -1 ¨ DE = -566. 0 k. J/mol – (8. 314 J/K*mol)(1 k. J/1000 J)(298 K)(-1) ¨ -563. 5 k. J/mol ¨
 Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Section 2 classifying chemical reactions
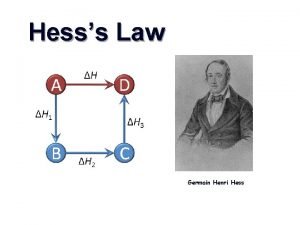
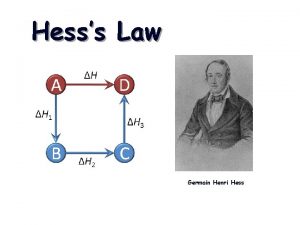
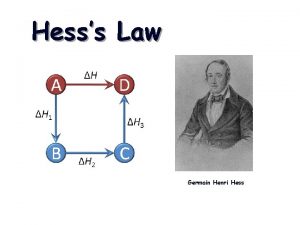
Section 2 classifying chemical reactions Hess's law rules
Hess's law rules Hesss law
Hesss law جرمان هنری هس
جرمان هنری هس Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Types of reactions
Types of reactions Chemical equations and reactions chapter 8 review
Chemical equations and reactions chapter 8 review Hesss
Hesss Section 1 atoms elements and compounds
Section 1 atoms elements and compounds Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Section 1 chemical changes
Section 1 chemical changes Are kc and kp equal
Are kc and kp equal Redox reactions half reactions
Redox reactions half reactions