Electrostatics Notes Charge n Have you ever walked




















- Slides: 35

Electrostatics Notes Charge!

n Have you ever walked across the carpet and gotten “shocked” when you touched the doorknob?

n What about static cling? Have you ever gotten to school only to be embarrassed when someone points out the sock sticking to your back?

What’s going on in these cases? Why did they occur?

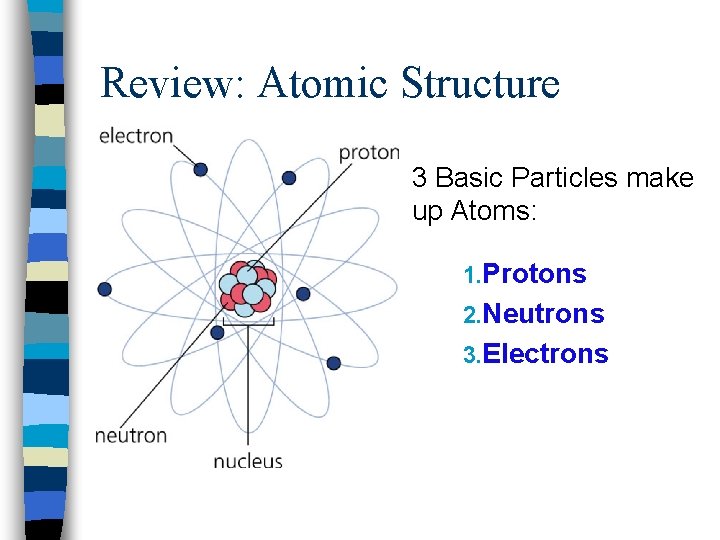
Review: Atomic Structure 3 Basic Particles make up Atoms: 1. Protons 2. Neutrons 3. Electrons

Charge n Protons & Electrons have a property called electric charge – Protons: positive electric charge (+) – Electrons: negative electric charge (-) – The strength of the positive charge on a proton is the same as the strength of the negative charge on the electron – Neutrons do not have charge: neutral

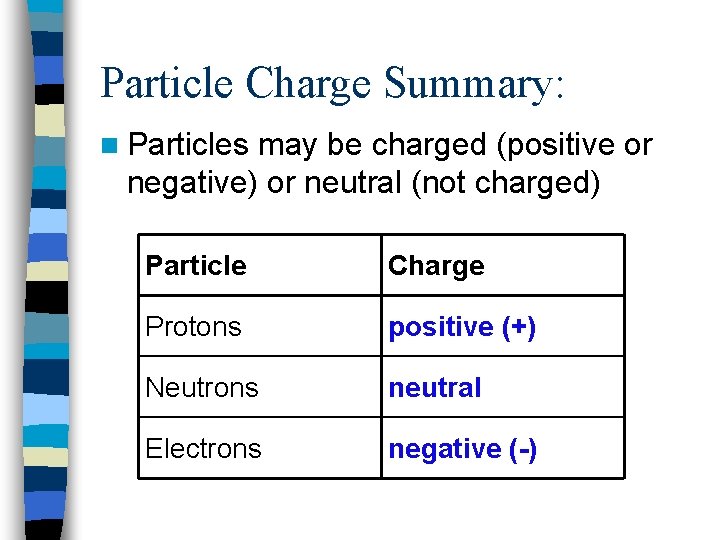
Particle Charge Summary: n Particles may be charged (positive or negative) or neutral (not charged) Particle Charge Protons positive (+) Neutrons neutral Electrons negative (-)

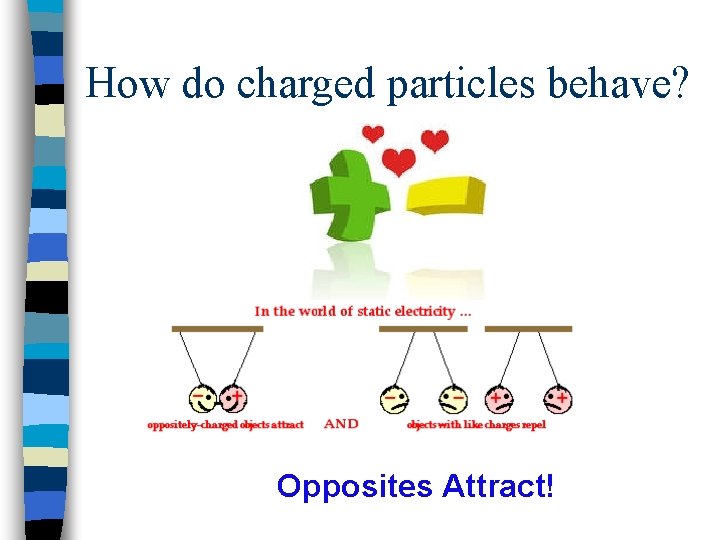
How do charged particles behave? Opposites Attract!

In the real world, we rarely deal with individual charged particles n Everyday objects are made of lots of atoms n Most atoms have an equal number of protons and electrons n Therefore, atoms are neutral – Remember, even though atoms are neutral, they are still made of charges

What does it mean to say that an object is neutral? n. A neutral object has no net (overall) charge n A neutral object has equal amounts of positive and negative charge

What does it mean to say that an object is charged? n. A charged object has a net charge n A positively charged object has a greater quantity of positive charge than negative charge n A negatively charged object has a greater quantity of negative charge than positive charge

Electrons move, Protons don’t! n Protons don’t move! – Protons are very massive. They have too much inertia. – They are in the center of the atom. n Electrons are outside the nucleus. – It is easier to move particles on the perimeter.

What do you have to do to make an object positively charged? electrons away from the object n You need to take

What do you have to do to make an object negatively charged? n You need to transfer the object. electrons to

Basically… n Oppositely charged particles attract each other – Ex: Protons (+) and Electrons (-) attract n Particles with the same charge repel each other – Ex: 2 Electrons (-) would repel each other – Ex: 2 Protons (+) would repel each other

Continued…. . n Particles with neutral charge do not interact – Neutrons do not attract or repel each other – Neutrons do not attract or repel electrons or protons

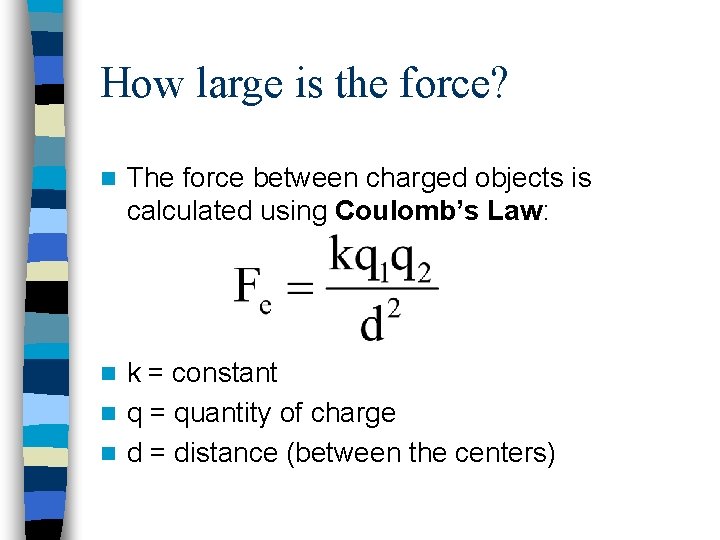
How large is the force? n The force between charged objects is calculated using Coulomb’s Law: k = constant n q = quantity of charge n d = distance (between the centers) n

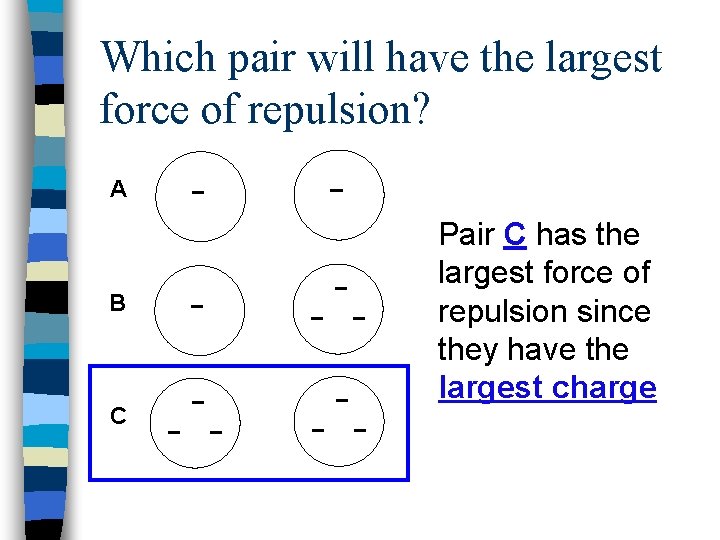
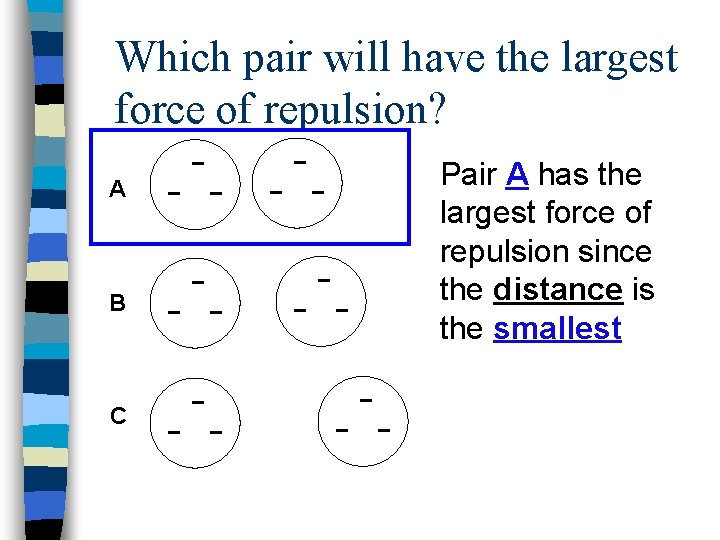
Which pair will have the largest force of repulsion? A B C Pair C has the largest force of repulsion since they have the largest charge

Which pair will have the largest force of repulsion? A B C Pair A has the largest force of repulsion since the distance is the smallest

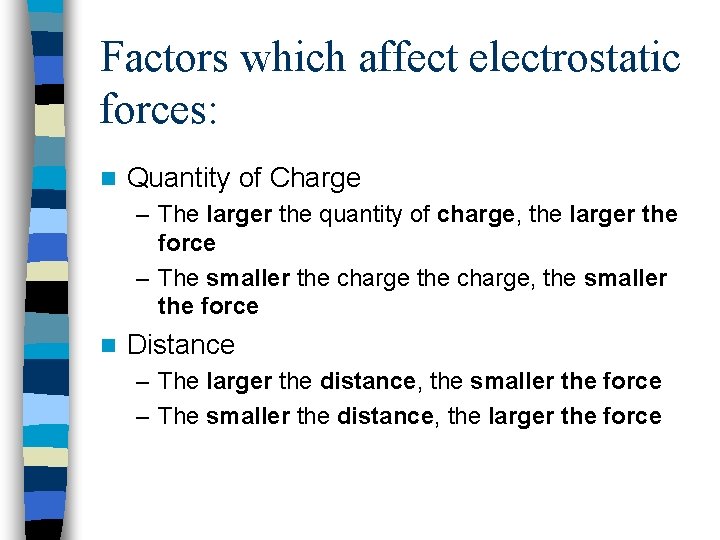
Factors which affect electrostatic forces: n Quantity of Charge – The larger the quantity of charge, the larger the force – The smaller the charge, the smaller the force n Distance – The larger the distance, the smaller the force – The smaller the distance, the larger the force

So, a charged sock can stick to my shirt… Does that mean that my shirt is charged? n Not necessarily n Remember that a neutral object is made up of innumerable positively and negatively charged particles. n A charged object (positive or negative) will be attracted to a neutral object. – We’ll discuss exactly why later…

Conductors vs. Insulators n Conductors: – Loosely bound electrons – Allow the flow of electrons – Examples: metals n Insulators: – Tightly bound electrons – Slow the flow of electric charge – Examples: rubber, plastic, and styrofoam

So what is happening when you rub a balloon on your head and it becomes charged? Is friction creating charge? n No! Charge cannot be created or destroyed. – Conservation of Charge n Charges are being exchanged…

Charging Methods n Objects can be charged by – Conduction – Induction – Friction (triboelectricity) – Polarization – Grounding

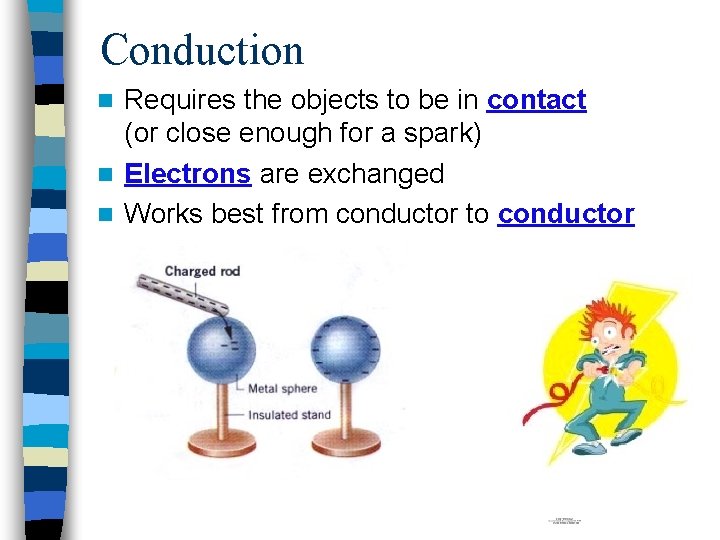
Conduction Requires the objects to be in contact (or close enough for a spark) n Electrons are exchanged n Works best from conductor to conductor n

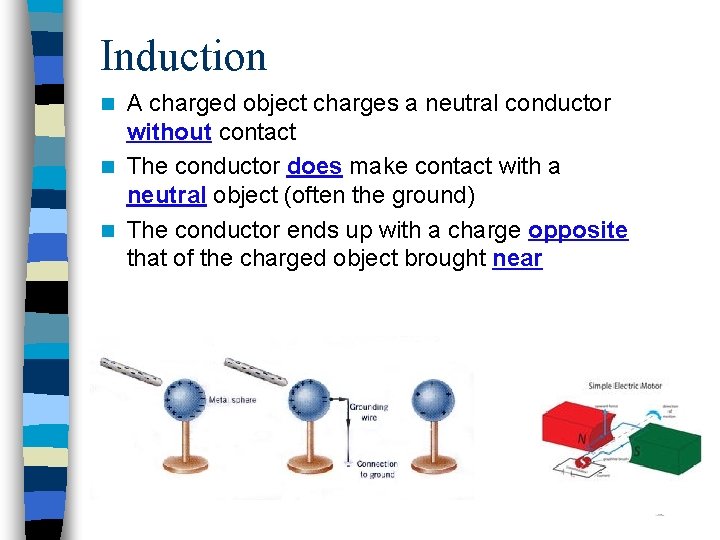
Induction A charged object charges a neutral conductor without contact n The conductor does make contact with a neutral object (often the ground) n The conductor ends up with a charge opposite that of the charged object brought near n

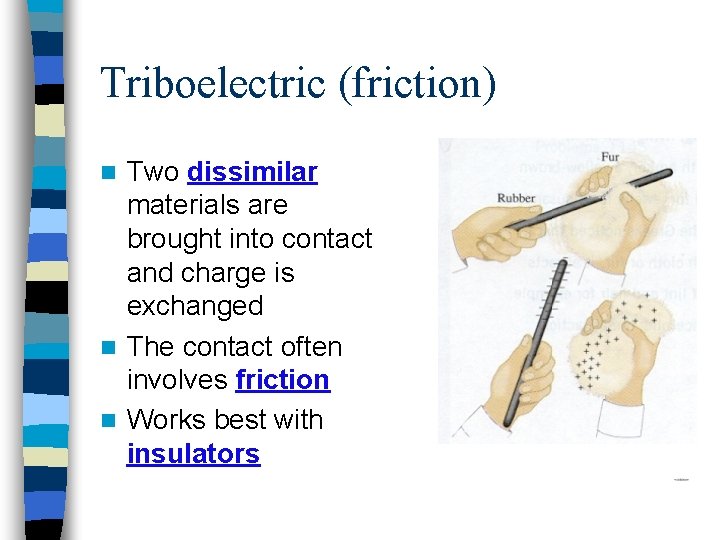
Triboelectric (friction) Two dissimilar materials are brought into contact and charge is exchanged n The contact often involves friction n Works best with insulators n

More on Triboelectricity n Charge separation occurs when two insulators are rubbed together n One of the insulators is more likely to grab electrons and the other insulator is more likely to donate electrons n CHARGE IS NOT CREATED! Electrons are simply being transferred.

The Triboelectric Series Rabbit fur Electron Donors Glass (objects that give electrons become positive) Wool Silk Cotton Amber Plastic (objects that take electrons become negative) Electron Grabbers

Triboelectric Charging You rub a balloon against your hair, and the hair becomes positively charged. This means that A. Electrons moved from the balloon to your hair. B. Protons moved from the balloon to your hair. C. Protons moved from your hair to the balloon. D. Electrons moved from your hair to the balloon. E. The rubbing destroyed electrons in your hair, leaving it positive.

Triboelectric Series A Triboelectric Sequence ELECTRON GRABBERS Rubber Amber Cotton ELECTRON DONORS Silk Cat fur Wool Glass Rabbit fur If you rub cotton with amber, which becomes positive? A. Amber B. Neither C. Cotton Which of the following can make glass negative? A. Amber B. Cat fur C. Rabbit fur

Polarization n As we said earlier, charged objects can be attracted to neutral objects – Charges can rearrange on neutral objects

Neutralizing/Grounding Objects n When a charged object comes in contact with a very large, neutral conductor, the object becomes neutralized. n Earth itself is a large, neutral conductor, so it neutralizes charged objects quite well.

Review n Charged objects exert forces: Like repels like Opposites attract Charged objects and neutral objects attract n How objects get charged or neutralized: – – – Conduction Induction Friction/Triboelectric Polarization Grounding

The End! HOORAY!!!