Electromagnetic Radiation Waves a review p Most waves


- Slides: 18

Electromagnetic Radiation

Waves… a review p Most waves are either longitudinal or transverse. p Sound waves are longitudinal. p But all electromagnetic waves are transverse…


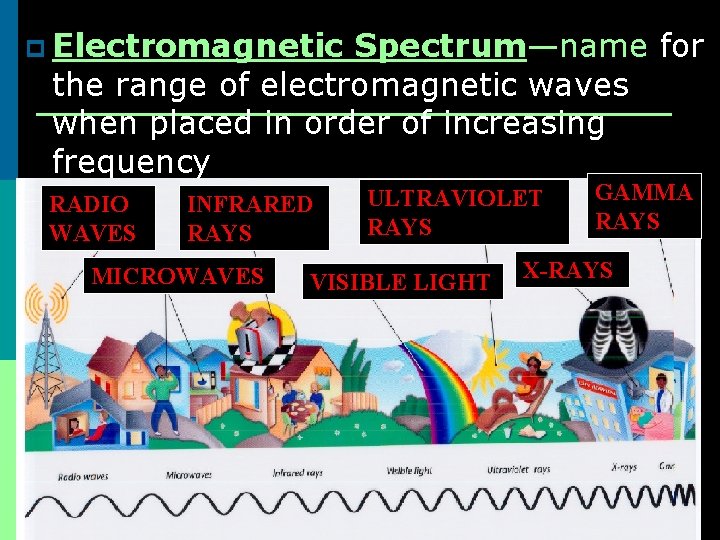
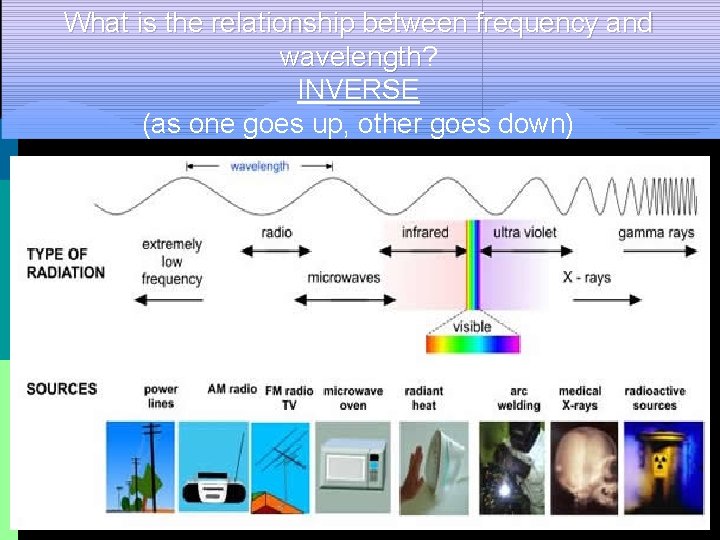
p Electromagnetic Spectrum—name for the range of electromagnetic waves when placed in order of increasing frequency RADIO WAVES INFRARED RAYS MICROWAVES ULTRAVIOLET RAYS VISIBLE LIGHT GAMMA RAYS X-RAYS

Electromagnetic waves p Produced by the movement of electrically charged particles p Can travel in a “vacuum” (they do NOT need a medium) p Travel at the speed of light c= 3. 0 x 108 m/s p Also known as EM waves

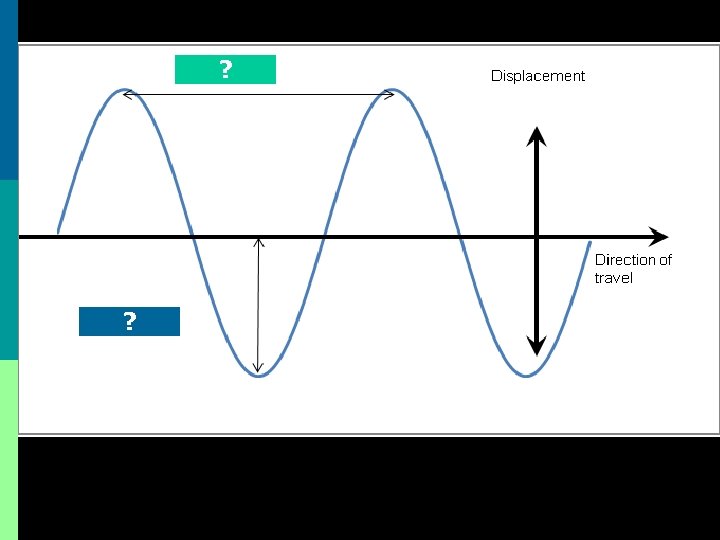
What is the relationship between frequency and wavelength? wavelength INVERSE (as one goes up, other goes down)

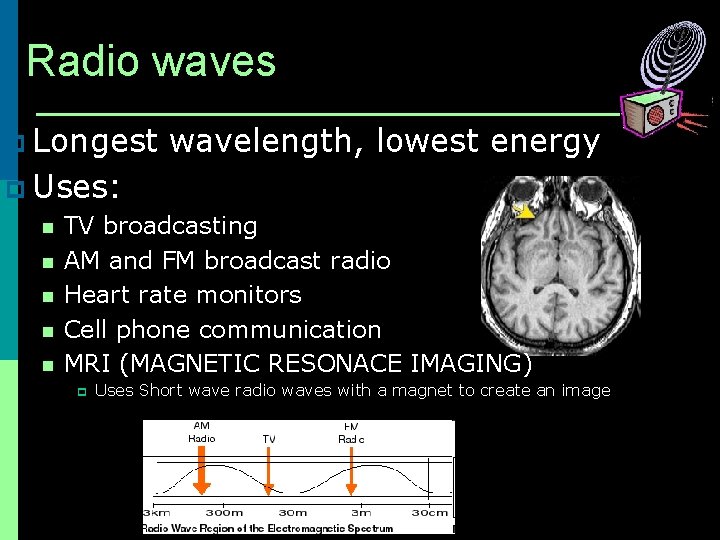
Radio waves p Longest wavelength, lowest energy p Uses: n TV broadcasting n AM and FM broadcast radio n Heart rate monitors n Cell phone communication n MRI (MAGNETIC RESONACE IMAGING) p Uses Short wave radio waves with a magnet to create an image

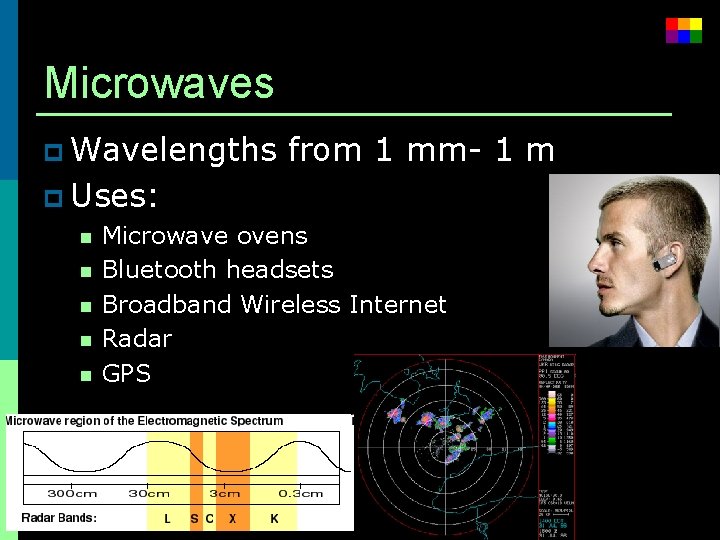
Microwaves p Wavelengths from 1 mm- 1 m p Uses: n Microwave ovens n Bluetooth headsets n Broadband Wireless Internet n Radar n GPS

Infrared Radiation p Wavelengths in between microwaves and visible light p Uses: n n n Night vision goggles Remote controls Heat-seeking missiles

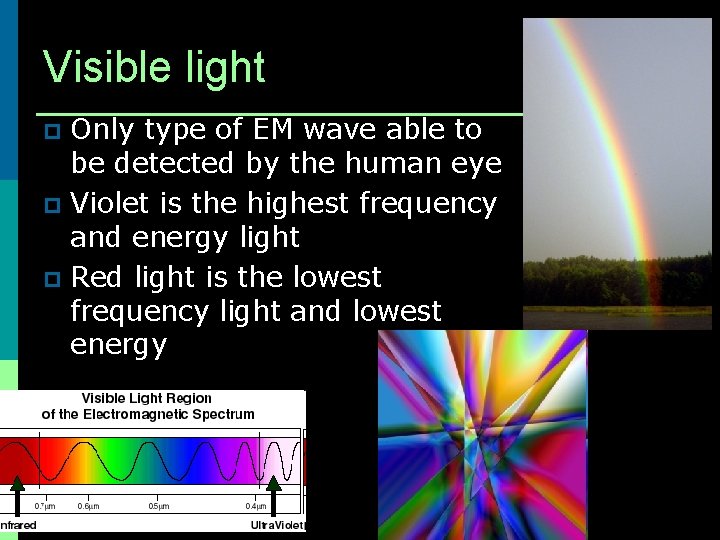
Visible light Only type of EM wave able to be detected by the human eye p Violet is the highest frequency and energy light p Red light is the lowest frequency light and lowest energy p

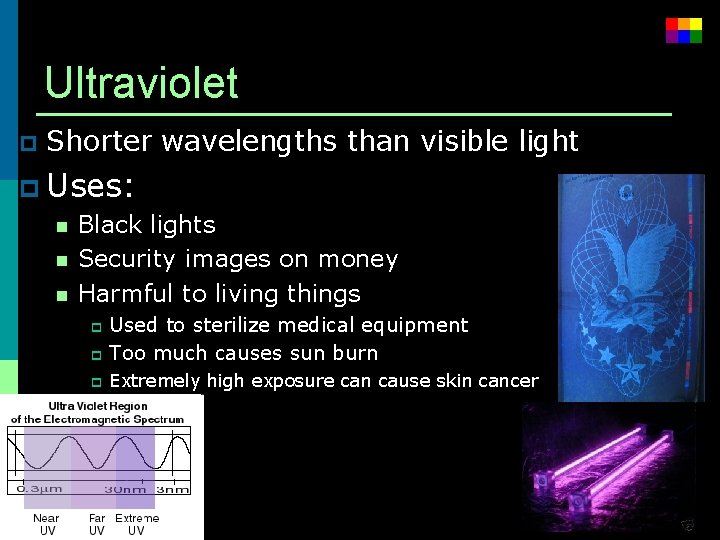
Ultraviolet p Shorter wavelengths than visible light p Uses: n Black lights n Security images on money n Harmful to living things Used to sterilize medical equipment p Too much causes sun burn p p Extremely high exposure can cause skin cancer

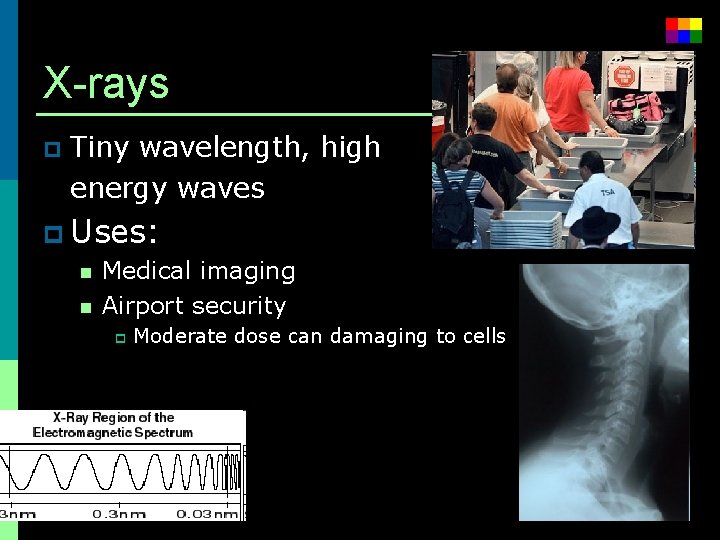
X-rays p Tiny wavelength, high energy waves p Uses: n Medical imaging n Airport security p Moderate dose can damaging to cells

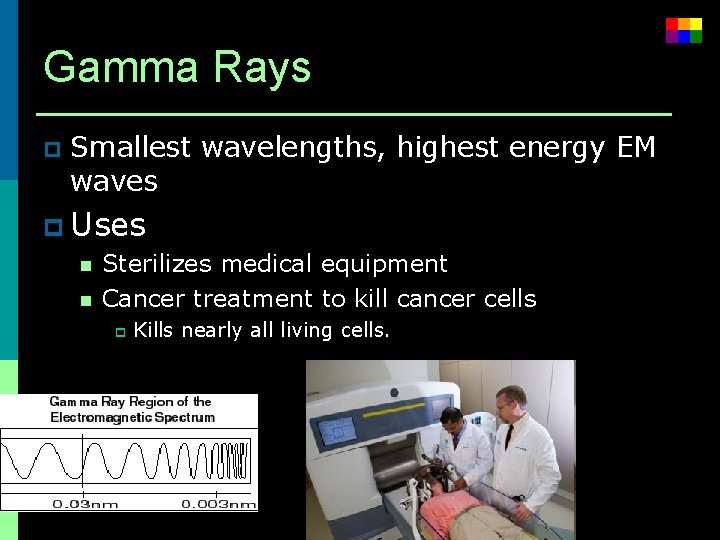
Gamma Rays p Smallest wavelengths, highest energy EM waves p Uses n Sterilizes medical equipment n Cancer treatment to kill cancer cells p Kills nearly all living cells.

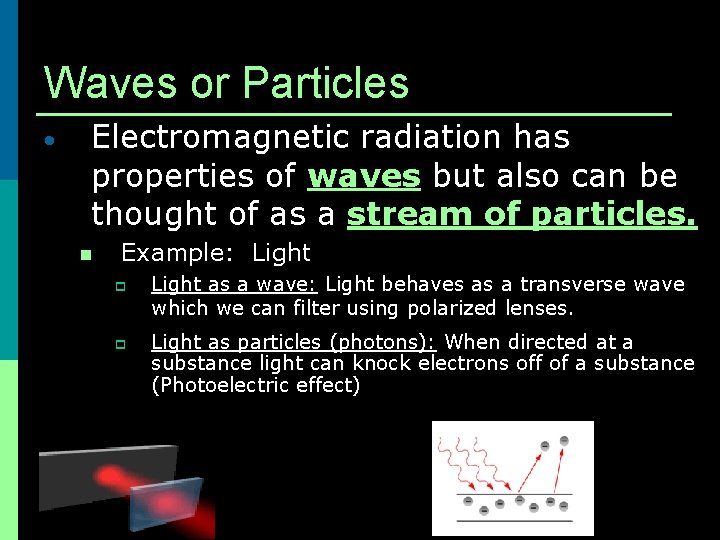
Waves or Particles • Electromagnetic radiation has properties of waves but also can be thought of as a stream of particles. n Example: Light p p Light as a wave: Light behaves as a transverse wave which we can filter using polarized lenses. Light as particles (photons): When directed at a substance light can knock electrons off of a substance (Photoelectric effect)

Emission of Light p Quantum – minimum amount of energy that can be gained or lost by an atom; can be referred to as a packet of energy p Photon – packet of light energy (quantum)

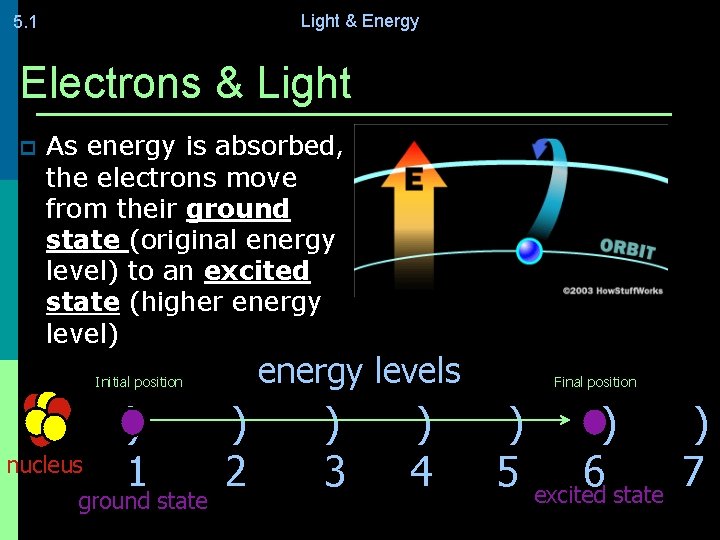
Light & Energy 5. 1 Electrons & Light p As energy is absorbed, the electrons move from their ground state (original energy level) to an excited state (higher energy level) energy levels Initial position nucleus ) 1 ground state ) 2 ) 3 ) 4 Final position ) ) ) 5 excited 6 state 7

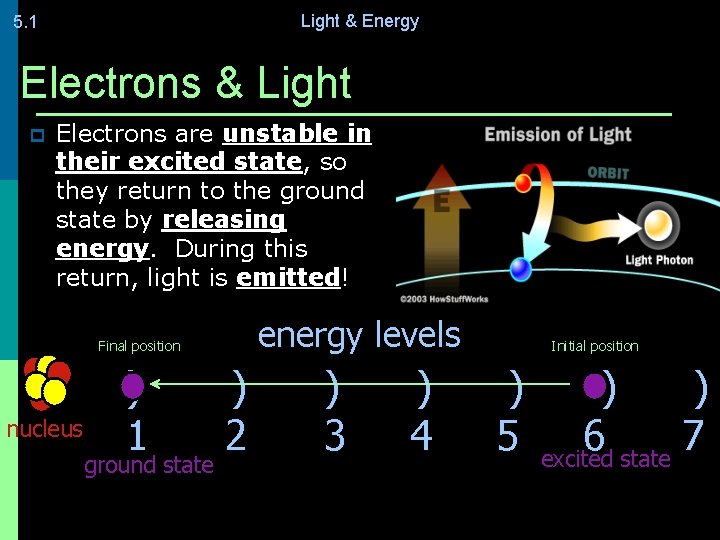
Light & Energy 5. 1 Electrons & Light p Electrons are unstable in their excited state, so they return to the ground state by releasing energy. During this return, light is emitted! energy levels Final position nucleus ) 1 ground state ) 2 ) 3 ) 4 Initial position ) 5 ) ) 6 7 excited state

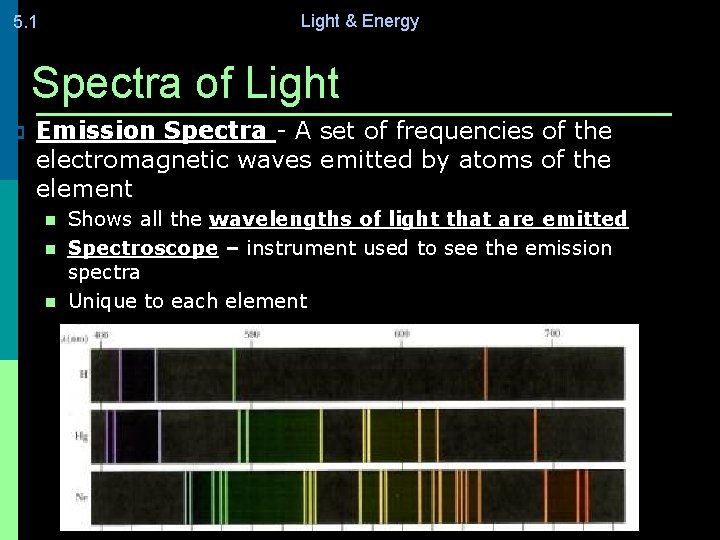
Light & Energy 5. 1 Spectra of Light p Emission Spectra - A set of frequencies of the electromagnetic waves emitted by atoms of the element n n n Shows all the wavelengths of light that are emitted Spectroscope – instrument used to see the emission spectra Unique to each element