DOT Hazardous Materials Regulated Medical Waste Nathan Douglas





































- Slides: 41

DOT Hazardous Materials Regulated Medical Waste Nathan Douglas, R. S. , M. S. Chemical & Biological Safety Officer Marshall University

Why are we here? “A person who knowingly violates a requirement of the Federal hazardous material transportation law, an order issued thereunder, … is liable for a civil penalty of not more than $50, 000 and not less than $250 for each violation, except the maximum civil penalty is $100, 000 if the violation results in death, serious illness or severe injury to any person or substantial destruction of property, and a minimum $450 civil penalty applies to a violation relating to training. When the violation is a continuing one, each day of the violation constitutes a separate offense. ” 49 CFR 107. 329

Training Outline General awareness / familiarization Function-specific training Safety training Personal Protection Accident Avoidance Emergency Response Security awareness and in-depth training 49 CFR 172. 704

Regulations Several agencies have regulations that cover Regulated Medical Waste (RMW) also called: biohazard waste, infectious medical waste, red bag trash, and regulated waste. Department of Transportation 49 CFR, subtitle B, Chapter 1, subchapter C Occupational Safety & Health Administration 29 CFR 1910. 1030 West Virginia Department of Health & Human Resources 64 CSR 56

Hazardous Material Employee A person who is employed, and in the course of such employment directly affects hazardous materials transportation safety Includes all employees that Load, unload, or handle hazardous materials; Prepare hazardous materials for transportation; Sign waste transport manifest papers 49 CFR 171. 8

Training Requirements Hazmat employees must be trained when performing duties Refresher training is required every 3 years Must be directly supervised by a trained person until trained Training conducted within 90 days of hire or assignment of duties 49 CFR 172 subpart H

What is a Hazardous Material (HAZMAT) According to the US DOT: A substance or material that the Secretary of Transportation has determined is capable of posing an unreasonable risk to health, safety, and property when transported in commerce, and has designated as hazardous. 49 CFR 171. 8

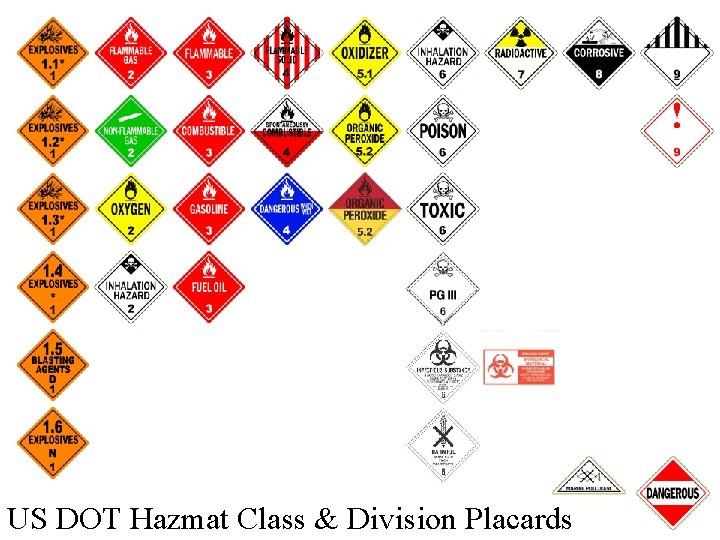
Hazardous Materials Dangerous goods are divided into 9 classes on the basis of the risk they present. They can be assigned more than 1 class if they present additional hazards Each class has a primary hazard, and can have numerous sub-hazards, called divisions. Vehicles transporting hazmat are required to display a placard indicating the hazard contained inside. 49 CFR 171. 8

US DOT Hazmat Class & Division Placards

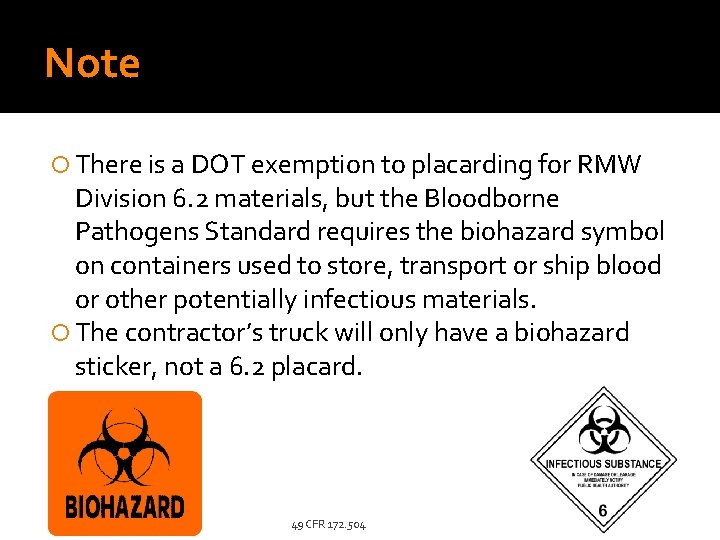
Note There is a DOT exemption to placarding for RMW Division 6. 2 materials, but the Bloodborne Pathogens Standard requires the biohazard symbol on containers used to store, transport or ship blood or other potentially infectious materials. The contractor’s truck will only have a biohazard sticker, not a 6. 2 placard. 49 CFR 172. 504

Hazardous Material Packaging Each Class & Division has established Packing Group requirements. Either PG I, II, or III The Packing Group indicates the degree of danger presented by the Hazmat, and determines the protective packaging required for safe transport PG I – great danger, most protective packaging PG II – medium danger PG III – least danger, least protective packaging 49 CFR 171. 8

Our Hazardous Materials Class 2 – Poisonous / Toxic Division 6. 2, Infectious Substances Category B Biological Products Cultures Patient Specimens Regulated Medical Wastes 49 CFR 173. 134(a)(1)-(4)

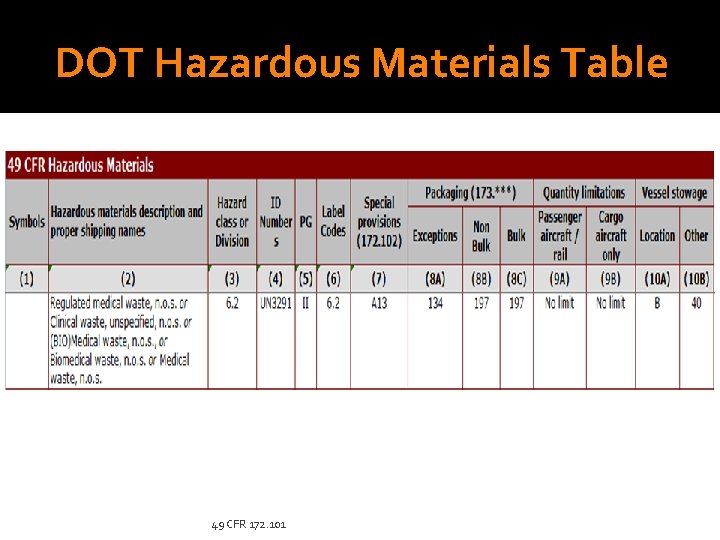
DOT Hazardous Materials Table 49 CFR 172. 101

DOT Hazardous Materials Table 1. Symbols: 2. Description and Proper Shipping Name: None Regulated medical waste, n. o. s. Clinical waste, unspecified, n. o. s. (BIO)Medical waste, n. o. s. , Biomedical waste, n. o. s. or Medical waste, n. o. s 3. Hazard Class or Division: 6. 2 4. Identification Numbers: UN 3291 5. Packing Group (PG): II 49 CFR 172. 101

Regulated Medical Waste A waste or reusable material derived from the medical treatment of an animal or human, which includes diagnosis and immunization, or from biomedical research, which includes the production and testing of biological products. 49 CFR 173. 134(a)(5)

OSHA – Regulated Waste Liquid or semi-liquid blood or other potentially infectious materials; contaminated items that would release blood or other potentially infectious materials in a liquid or semi-liquid state if compressed; items that are caked with dried blood or other potentially infectious materials and are capable of releasing these materials during handling; contaminated sharps; and pathological and microbiological wastes containing blood or other potentially infectious materials. 29 CFR 1910. 1030(b)

WVDHHR – Infectious Medical Waste Medical waste which is capable of producing an infectious disease. Medical waste shall be considered capable of producing an infectious disease if it has been, or is likely to have been, contaminated by an organism likely to be pathogenic to healthy humans, if such organism is not routinely and freely available in the community, and such organism has a significant probability of being present in sufficient quantities and with sufficient virulence to transmit disease. 64 CSR 56. 3. 9.

Sharps Any object contaminated with a pathogen or that may become contaminated with a pathogen through handling or during transportation and also capable of cutting or penetrating skin or a packaging material. Includes needles, syringes, scalpels, broken glass, culture slides, culture dishes, broken capillary tubes, broken rigid plastic, and exposed ends of dental wires. 49 CFR 173. 134(a)(6)

Package the Material Regulated medical waste must be packaged at the Packing Group II performance level and meet general packaging requirements PG II exception for private/contract carriers Inner containers must be packed with closures upward if they contain liquid hazardous materials 49 CFR 172. 312 / 49 CFR 173. 197

Packaging Requirements Inner bag with minimum thickness of 1. 5 mil Sharps containers must be securely closed to prevent spills or protrusions Top of bag closed by tying in a knot; or twisting and folding over, then tightly taping Shipping container snapped closed or cardboard box taped shut on all seams 49 CFR 173. 24

Packaging Requirements Package filled and its contents limited so under normal conditions of transportation there is no: release of hazardous material to the environment substantial reduction in the effectiveness of the package chemical reaction with or inside the package breakage, leakage or movement of the inner packaging 49 CFR 173. 24

Additional Requirements Items like staples do not protrude through the outer packaging in a way that would damage the inner packaging. The package is not filled to a gross mass greater than the maximum gross marked on the packaging (Standard 18 x 22 boxes are limited to 45 lbs). No hazardous material may remain on the outside of a package after filling. A package containing inner packagings of Division 6. 2 materials may not contain other hazardous materials. 49 CFR 173. 24 a

Package Markings General marking requirements: Proper Shipping Name and Identification Number Package Orientation Arrows Generating Facility’s Name and Address WV Requires the package to be dated once full Markings must be: Durable, in English, and printed on the surface or on a label, tag, or sign Displayed on a contrasting background Unobscured by labels or attachments 49 CFR 172. 304

Biohazard Labeling in the Bloodborne Pathogens Std. Warning labels shall be affixed to containers of regulated waste. Labels have to have biohazard symbol & wording per OSHA Bloodborne Pathogen Standard: 29 CFR 1910. 1030(g)(1)(i)

Pathologic Waste Stericycle uses a labeling system to differentiate between packages of Regulated Medical Waste that can be autoclaved and those that must be incinerated. Pathologic and trace chemotherapeutic wastes must be incinerated. We should use incinerator-only for pharmaceuticals. Our Generator Label is typically white. Yellow labels are used for incinerate-only waste.

Manifest - Shipping Papers All manifests will have the following: Identification Number (UN 3291) Proper Shipping Name (Regulated Medical Waste, n. o. s. ) Hazard Class or Division (6. 2) Packing Group (PG II) Description of the shipping container The manifest must also include, the total quantity of material shipped. 49 CFR 172. 202

Manifest – Shipping Papers Quantity of Materials Shipped Usually report number of containers and volume in cubic feet ▪ Boxes are generally 4. 3 cu ft. Can also be reported by weight per box, and total You should verify that the total quantity shipped block is the same as the number of boxes removed from the facility 49 CFR 172. 202

Completing the Manifests also contain “Shipper’s Certification” This is to certify that the above named materials are properly classified, described, packaged, marked and labeled, and are in proper condition for transportation according to the applicable regulations of the Department of Transportation. Must be signed legibly by a trained employee. Indicates that we agree with the volume (number of containers) of waste sent off-site 49 CFR 172. 204

Safety OSHA Bloodborne Pathogen Standard Mandates that employers provide a safe and healthy work environment Provide Hepatitis B vaccination Provide required PPE and ensure it is used Provide training on HBV and HIV and other bloodborne pathogens annually Have a written Exposure Control Plan, revised annually, evaluate safer sharps systems and use them where feasible

Personal Protection OSHA Bloodborne Pathogen Standard Requires employees to observe Universal Precautions ▪ Treat all contaminated items like they’re infectious ▪ Wear appropriate PPE ▪ Handle and dispose properly Healthcare personnel have 2 main PPE ▪ exam gloves and clothing (scrubs). Face masks used where splashes/sprays are anticipated, must also use safety glasses/goggles

Accident Avoidance Most common healthcare accident is needlesticks Extra care must be exhibited when working with children that squirm Sharps containers must be replaced when contents reach the “full” line, not allowed to overfill Containers must be securely closed before they’re moved out of the exam room

Emergency Response 1 st – Needlestick Follow Exposure Control Plan – available on employee web site ▪ Wash the affected area with soap and water ▪ Notify department Collateral Duty Safety Officer ▪ They will identify the source patient for testing, obtain consent, and have specimen collected ▪ Immediately seek treatment at nearest Emergency Department ▪ Identify yourself as having been exposed to BBP ▪ Prophylaxis should be given within 1 -2 hours ▪ Request a Workers Compensation form be completed, not insurance

Emergency Response Needlestick, cont ▪ Follow up with Internal Medicine Department ▪ They will discuss lab reports from source patient and exposed employee ▪ Provide consultation about potential diseases and recommended testing timeline ▪ Complete a Needlestick Incident Report and submit to Safety Officer ▪ Safety Officer will ensure information is complete and will review incident with post-exposure management team, and maintain records

Emergency Response 2 nd – Spill of blood or OPIM For small spill (1 vaccutainer or less, 10 ml max) Cleanup should only be performed by persons trained in Bloodborne Pathogens Wear gloves Spray area with disinfectant ▪ Fresh bleach 1: 10 dilution , or tuberculocidal product Use tongs or other mechanical means to pick up sharps and broken glass. Dispose in sharps box.

Emergency Response Small Spill of Blood or OPIM, cont. Wipe up liquids with paper towel and dispose in biohazard bag. Spray the area of the spill with disinfectant again Allow 15 minutes contact time, or per instructions on container if duration is different Wash and disinfect mechanical devices used during cleanup Dispose gloves in biohazard bag

Emergency Response Spill of Blood or OPIM For large spill (more than 1 vaccutainer, +10 ml) Secure the area from entry by unauthorized persons Put on appropriate PPE (gown, shoe covers, mask, etc. ) Spray all containers and the entire spill area with disinfectant and allow 15 minutes contact time.

Emergency Response Large Spill of Blood or OPIM Clean up sharps and liquids similar to small spill Disinfect area again, allow 15 minutes contact time, or per label instructions Clean and disinfect equipment Dispose single-use PPE. Biohazard if soiled, trash if not. Make arrangements to have spill kit replenished, as necessary.

Security Marshall University, the Joan C. Edwards School of Medicine, University Physicians & Surgeons, Inc. and the Marshall University Medical Center manage Regulated Medical Waste in a controlled manner where only authorized personnel have access to secured storage areas. Due to the nature of Regulated Medical Waste generation patients are in areas where waste is initially generated and temporarily stored. Waste is either treated on-site via autoclave or shipped offsite through a permitted contract service, documented by waste manifests that are retained for 3 years (WV requirement, DOT is 2 years).

Security Potential Threats Non-secured areas, or unattended points of entry Unauthorized personnel allowed access Prevention Techniques Know the driver(s) Look for an official uniform, ask for id when in doubt Report suspicious behavior

Compliance Checklist Inner container (bag) is closed. Box is in good condition. Box does not weigh more than 55 lbs. Outside of box is not contaminated. Stericycle barcode sticker is attached, and date filled in when box/bin taped closed. Total quantity is written on shipping paper. Shipping manifest is legibly signed by trained employee.

Conclusion DOT regulates the transportation of hazardous materials Proper identification Classification Packaging Training of personnel If you have questions, ask. Nathan Douglas, 696 -3461 ▪ douglas 2@marshall. edu
 Rmw transport container must contain
Rmw transport container must contain Dot regulated medical waste training
Dot regulated medical waste training Nj medical waste tracking form
Nj medical waste tracking form Regulated medical waste n o s
Regulated medical waste n o s Regulated medical waste n o s
Regulated medical waste n o s Regulated medical waste n o s
Regulated medical waste n o s Osha regulated medical waste
Osha regulated medical waste Id root
Id root Section 3 hazardous waste answers
Section 3 hazardous waste answers Biomedical waste definition
Biomedical waste definition Msw apes
Msw apes Example of hazardous waste
Example of hazardous waste Stanislaus county hazardous waste
Stanislaus county hazardous waste Waste management references
Waste management references Solid and hazardous waste
Solid and hazardous waste Hazardous waste transportation
Hazardous waste transportation Rcra hazardous waste refresher
Rcra hazardous waste refresher 49 cfr parts 171-179
49 cfr parts 171-179 Hazardous materials table
Hazardous materials table Hazardous
Hazardous Pa-psfa-hazardous materials awareness
Pa-psfa-hazardous materials awareness Define whmis
Define whmis Isachmm
Isachmm Hazardous materials transportation act of 1975
Hazardous materials transportation act of 1975 Hazardous materials managing the incident
Hazardous materials managing the incident Hazardous materials reference books
Hazardous materials reference books Pipeline and hazardous materials administration
Pipeline and hazardous materials administration Hazardous materials incident report
Hazardous materials incident report Hazardous materials business plan
Hazardous materials business plan Hazardous materials managing the incident
Hazardous materials managing the incident 29 cfr 1910 hazardous materials
29 cfr 1910 hazardous materials Open dot and closed dot on a graph
Open dot and closed dot on a graph Dot net vs dot com
Dot net vs dot com 10 chapter of nabh
10 chapter of nabh Cwtf in bio-medical waste
Cwtf in bio-medical waste Introduction of bio medical waste
Introduction of bio medical waste Hcrw management
Hcrw management Cbwtf full form
Cbwtf full form Frank duvergne
Frank duvergne How is citric acid cycle regulated
How is citric acid cycle regulated Mean arterial pressure
Mean arterial pressure Explain how the hts is regulated
Explain how the hts is regulated