Do we underutilize atherectomy Design and Rationale of








- Slides: 20

Do we underutilize atherectomy? Design and Rationale of the ECLIPSE Trial Sunil V. Rao MD

Disclosures n Previous 12 months l Amgen, Boehringer Ingelheim, CSI Inc. , Corindus, Medtronic n Thanks to Ajay Kirtane MD for slides

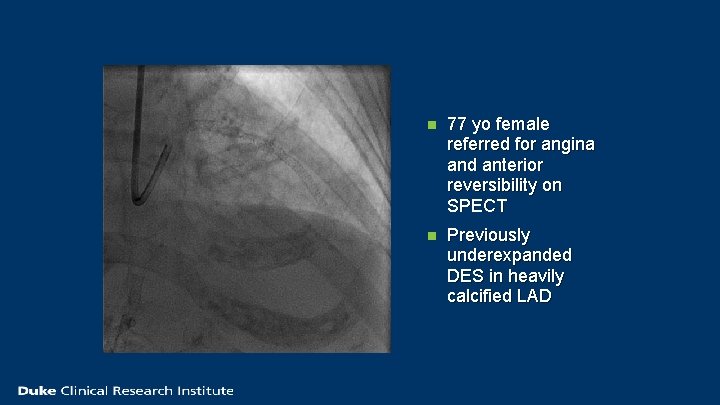
n 77 yo female referred for angina and anterior reversibility on SPECT n Previously underexpanded DES in heavily calcified LAD

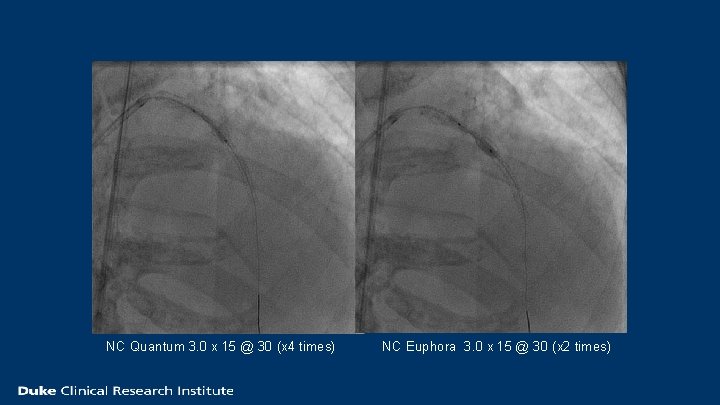
NC Quantum 3. 0 x 15 @ 30 (x 4 times) NC Euphora 3. 0 x 15 @ 30 (x 2 times)

Calcification – the interventionalist’s nemesis n Calcification of coronary lesions is common n Calcification limits procedure success n Calcification increases PCI complications

Rotational atherectomy Indication For Use: “the device can be used as a sole therapy or with adjunctive balloon angioplasty in patients with coronary artery disease who are candidates for coronary artery bypass graft surgery. ”

Orbital atherectomy – FDA approved for calcification The DIAMONDBACK 360 Coronary Orbital Atherectomy System (OAS) is a percutaneous orbital atherectomy system indicated to facilitate stent delivery in patients with coronary artery disease (CAD) who are acceptable candidates for PTCA or stenting due to de novo, severely calcified coronary artery lesions.

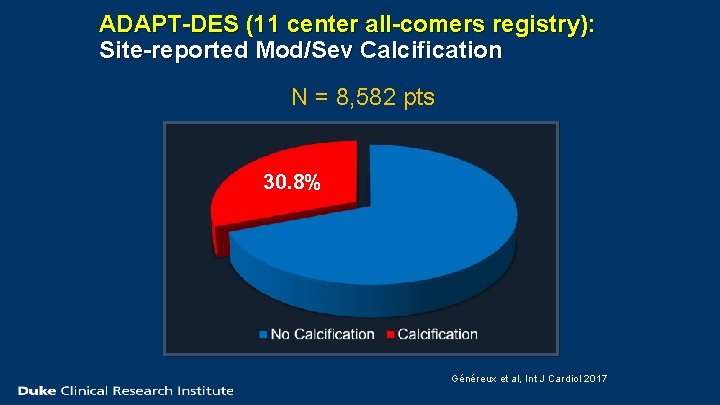
ADAPT-DES (11 center all-comers registry): Site-reported Mod/Sev Calcification N = 8, 582 pts 30. 8% Généreux et al, Int J Cardiol 2017

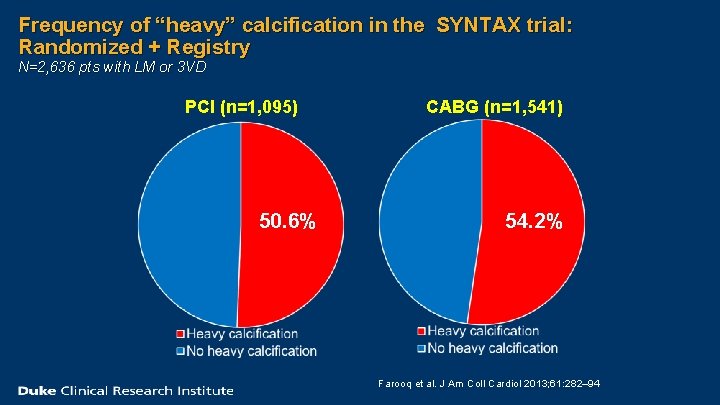
Frequency of “heavy” calcification in the SYNTAX trial: Randomized + Registry N=2, 636 pts with LM or 3 VD PCI (n=1, 095) 50. 6% CABG (n=1, 541) 54. 2% Farooq et al. J Am Coll Cardiol 2013; 61: 282– 94

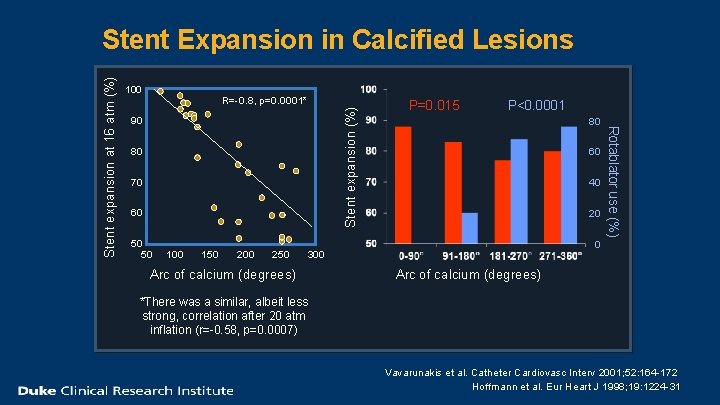
100 Stent expansion (%) R=-0. 8, p=0. 0001* 90 80 70 60 50 50 100 150 200 250 P=0. 015 P<0. 0001 80 60 40 20 0 300 Arc of calcium (degrees) Rotablator use (%) Stent expansion at 16 atm (%) Stent Expansion in Calcified Lesions Arc of calcium (degrees) *There was a similar, albeit less strong, correlation after 20 atm inflation (r=-0. 58, p=0. 0007) Vavarunakis et al. Catheter Cardiovasc Interv 2001; 52: 164 -172 Hoffmann et al. Eur Heart J 1998; 19: 1224 -31

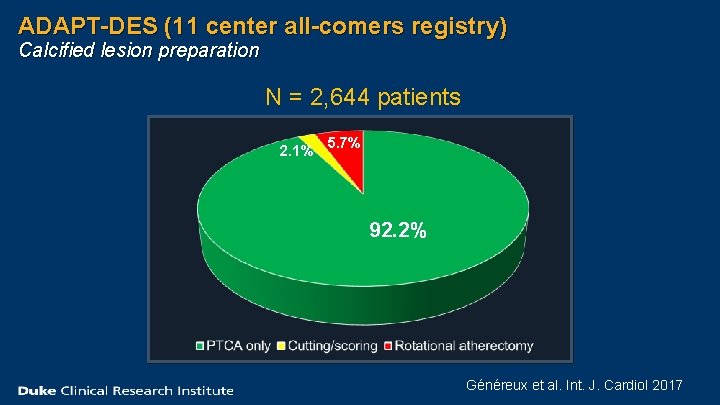
ADAPT-DES (11 center all-comers registry) Calcified lesion preparation N = 2, 644 patients 2. 1% 5. 7% 92. 2% Généreux et al. Int. J. Cardiol 2017

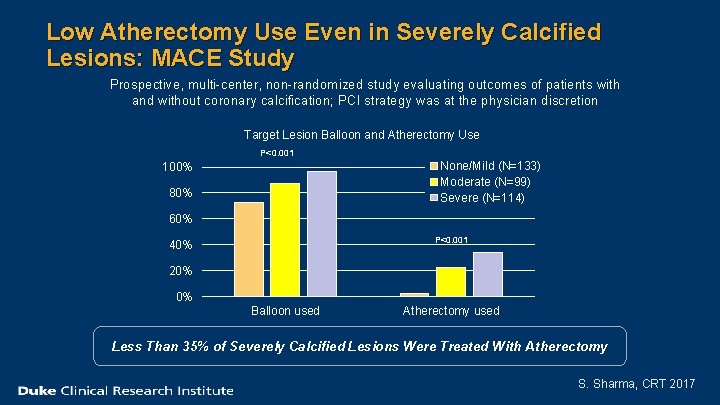
Low Atherectomy Use Even in Severely Calcified Lesions: MACE Study Prospective, multi-center, non-randomized study evaluating outcomes of patients with and without coronary calcification; PCI strategy was at the physician discretion Target Lesion Balloon and Atherectomy Use P<0. 001 None/Mild (N=133) Moderate (N=99) Severe (N=114) 100% 80% 60% P<0. 001 40% 20% 0% Balloon used Atherectomy used Less Than 35% of Severely Calcified Lesions Were Treated With Atherectomy S. Sharma, CRT 2017

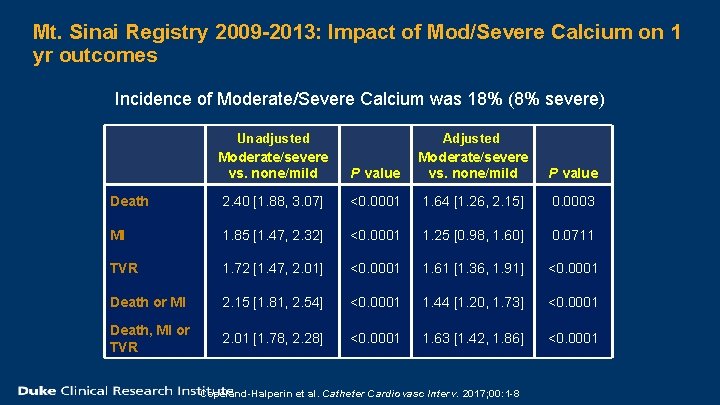
Mt. Sinai Registry 2009 -2013: Impact of Mod/Severe Calcium on 1 yr outcomes Incidence of Moderate/Severe Calcium was 18% (8% severe) Unadjusted Moderate/severe vs. none/mild P value Adjusted Moderate/severe vs. none/mild P value Death 2. 40 [1. 88, 3. 07] <0. 0001 1. 64 [1. 26, 2. 15] 0. 0003 MI 1. 85 [1. 47, 2. 32] <0. 0001 1. 25 [0. 98, 1. 60] 0. 0711 TVR 1. 72 [1. 47, 2. 01] <0. 0001 1. 61 [1. 36, 1. 91] <0. 0001 Death or MI 2. 15 [1. 81, 2. 54] <0. 0001 1. 44 [1. 20, 1. 73] <0. 0001 Death, MI or TVR 2. 01 [1. 78, 2. 28] <0. 0001 1. 63 [1. 42, 1. 86] <0. 0001 Copeland-Halperin et al. Catheter Cardiovasc Interv. 2017; 00: 1 -8

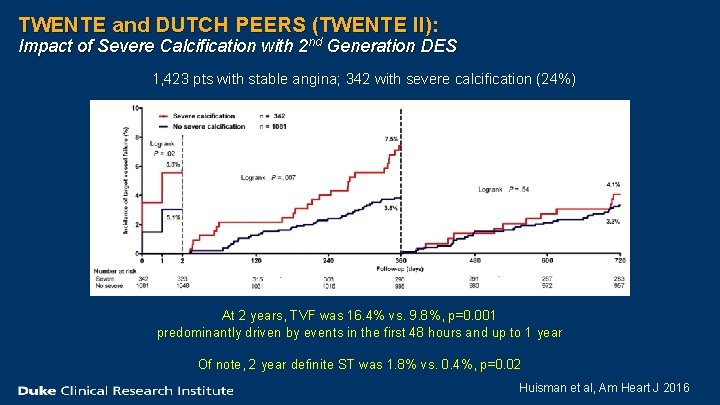
TWENTE and DUTCH PEERS (TWENTE II): Impact of Severe Calcification with 2 nd Generation DES 1, 423 pts with stable angina; 342 with severe calcification (24%) At 2 years, TVF was 16. 4% vs. 9. 8%, p=0. 001 predominantly driven by events in the first 48 hours and up to 1 year Of note, 2 year definite ST was 1. 8% vs. 0. 4%, p=0. 02 Huisman et al, Am Heart J 2016

If atherectomy is underutilized, what is the reason?

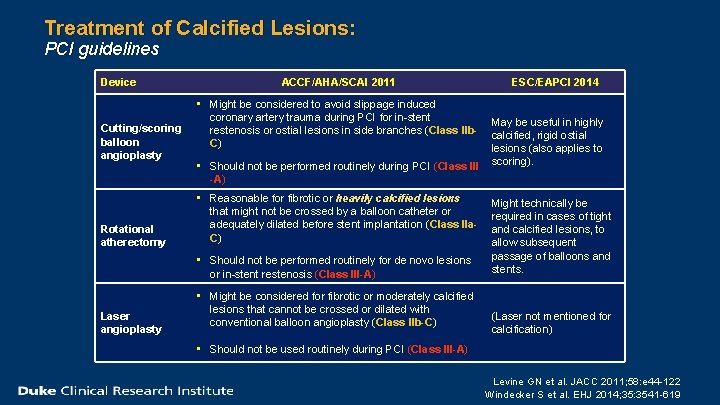
Treatment of Calcified Lesions: PCI guidelines Device Cutting/scoring balloon angioplasty Rotational atherectomy ACCF/AHA/SCAI 2011 • Might be considered to avoid slippage induced coronary artery trauma during PCI for in-stent restenosis or ostial lesions in side branches (Class IIb. C) • Should not be performed routinely during PCI (Class III -A) • Reasonable for fibrotic or heavily calcified lesions that might not be crossed by a balloon catheter or adequately dilated before stent implantation (Class IIa. C) • Should not be performed routinely for de novo lesions or in-stent restenosis (Class III-A) Laser angioplasty • Might be considered for fibrotic or moderately calcified lesions that cannot be crossed or dilated with conventional balloon angioplasty (Class IIb-C) ESC/EAPCI 2014 May be useful in highly calcified, rigid ostial lesions (also applies to scoring). Might technically be required in cases of tight and calcified lesions, to allow subsequent passage of balloons and stents. (Laser not mentioned for calcification) • Should not be used routinely during PCI (Class III-A) Levine GN et al. JACC 2011; 58: e 44 -122 Windecker S et al. EHJ 2014; 35: 3541 -619

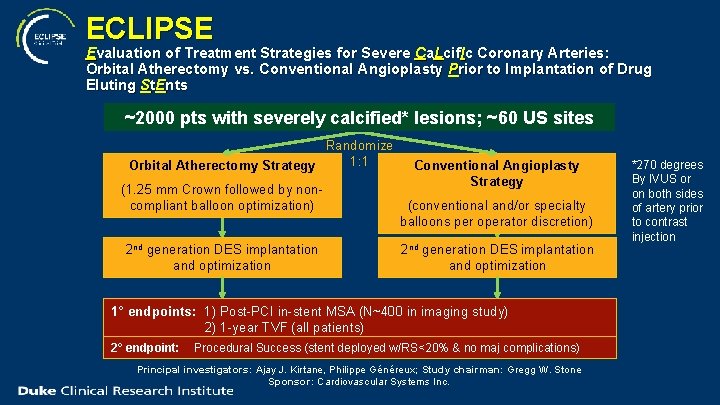
ECLIPSE Evaluation of Treatment Strategies for Severe Ca. Lcif. Ic Coronary Arteries: Orbital Atherectomy vs. Conventional Angioplasty Prior to Implantation of Drug Eluting St. Ents ~2000 pts with severely calcified* lesions; ~60 US sites Randomize 1: 1 Orbital Atherectomy Strategy (1. 25 mm Crown followed by noncompliant balloon optimization) 2 nd generation DES implantation and optimization Conventional Angioplasty Strategy (conventional and/or specialty balloons per operator discretion) 2 nd generation DES implantation and optimization 1° endpoints: 1) Post-PCI in-stent MSA (N~400 in imaging study) 2) 1 -year TVF (all patients) 2° endpoint: Procedural Success (stent deployed w/RS<20% & no maj complications) Principal investigators: Ajay J. Kirtane, Philippe Généreux; Study chairman: Gregg W. Stone Sponsor: Cardiovascular Systems Inc. *270 degrees By IVUS or on both sides of artery prior to contrast injection

ECLIPSE Trial: Challenges n Proficiency with device n Investigator equipoise n Treatment crossovers

ECLIPSE Trial: Summary n Calcification remains an unaddressed challenge in PCI n Rates of atherectomy are low in clinical practice n The ECLIPSE Trial is a large trial comparing atherectomy with balloon angioplasty in patients with calcified coronary lesions n Results from the ECLIPSE should inform clinical practice and treatment guidelines

Duke Univ. Medical Center Thank you. Duke Clinical Research Institute