DO NOW Pick up notes sheets If you











- Slides: 40

DO NOW § Pick up notes sheets. § If you did not turn in your Survey of the Elements lab Friday, turn it in now please.

PERIODIC TRENDS OR WHY WE CALL IT THE PERIODIC TABLE


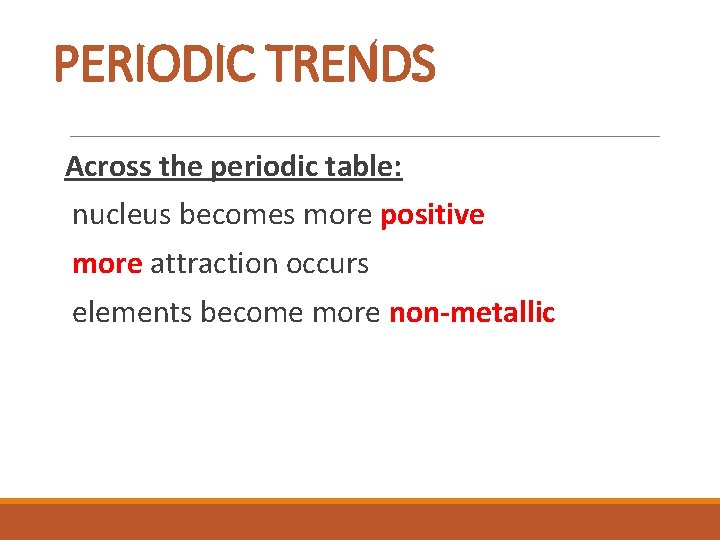
PERIODIC TRENDS Across the periodic table: nucleus becomes more positive more attraction occurs elements become more non-metallic

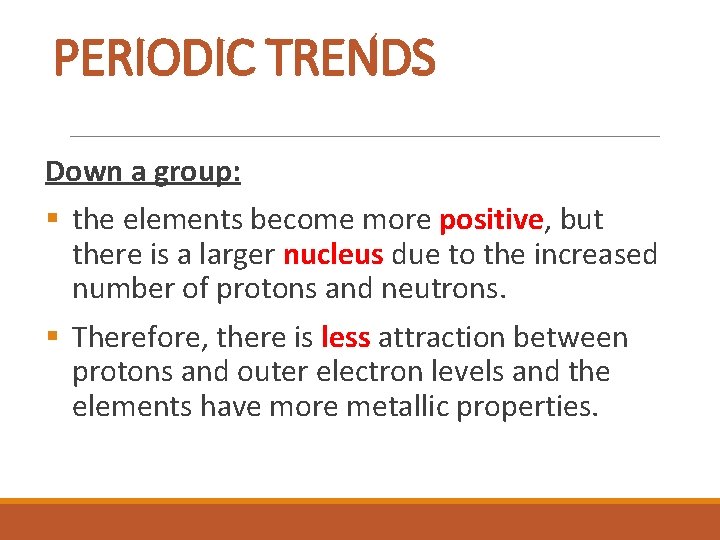
PERIODIC TRENDS Down a group: § the elements become more positive, but there is a larger nucleus due to the increased number of protons and neutrons. § Therefore, there is less attraction between protons and outer electron levels and the elements have more metallic properties.

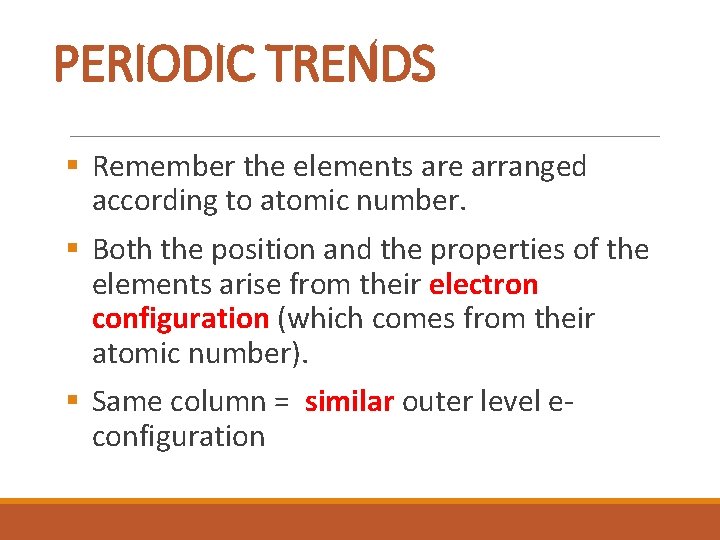
PERIODIC TRENDS § Remember the elements are arranged according to atomic number. § Both the position and the properties of the elements arise from their electron configuration (which comes from their atomic number). § Same column = similar outer level econfiguration

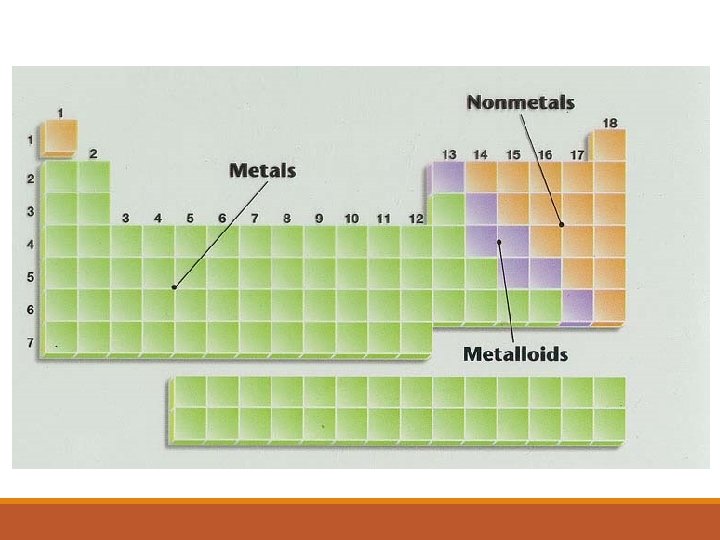
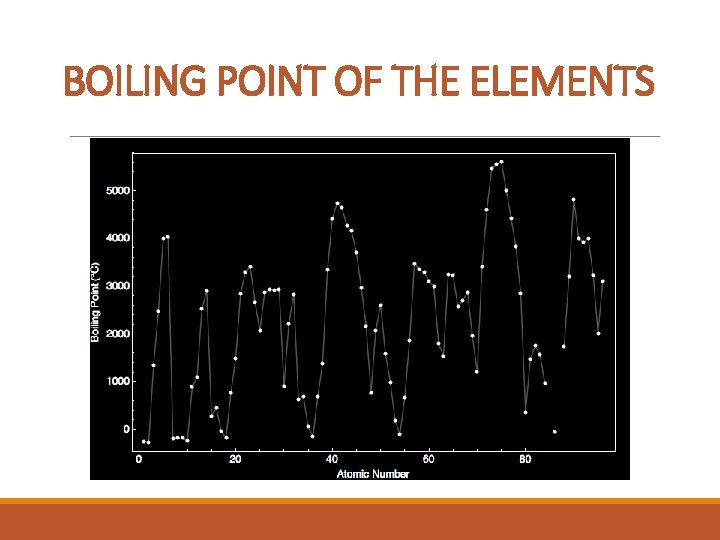
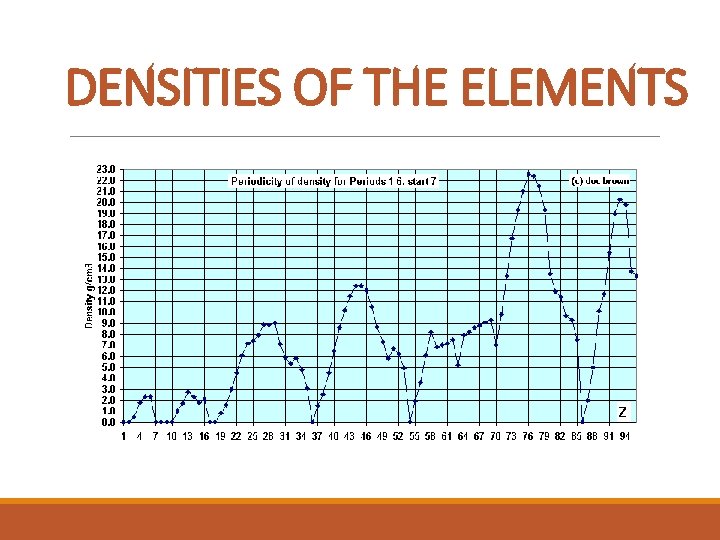
PERIODIC TRENDS Properties that are periodic: metals, metalloids, nonmetals, boiling point, density, atomic radii, ionization energies, electron affinities, electronegativity

BOILING POINT OF THE ELEMENTS

DENSITIES OF THE ELEMENTS

ALKALI METALS VIDEO Looking at a periodic trend of the alkali metal family.

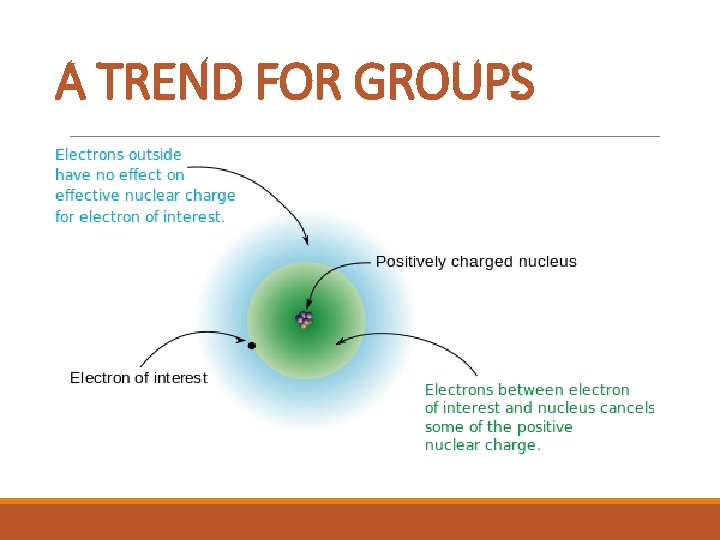
A TREND FOR GROUPS SHIELDING § The higher the period number, the more energy levels there are. § The orbitals are further out. § Inner electrons shield the positive charge of the protons and the electrons in the outer energy level come off more easily.

A TREND FOR GROUPS

A TREND FOR PERIODS PROTON PULL (nuclear charge) § The greater the positive charge (due to increased atomic number), the greater the attraction (pull) within the energy level. § More protons can be attracted to more electrons.

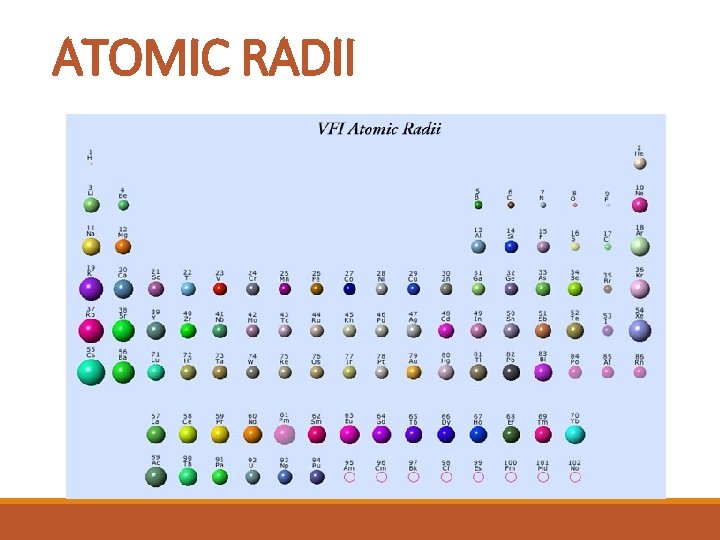
ATOMIC RADII § The size of the atom, measured by the radius. § As the energy levels increase, the period number increases , and so does the atomic radii. § As the atomic number increases across a period, the positive charge also increases within the same energy level.

ATOMIC RADII

ATOMIC RADII TREND: § atomic size increases down a group § atomic size decreases across a period. Why: within a group, inner level electrons shield outer level e- from the positive nucleus; distance from nucleus increases; within a period, size of nuclear charge increases and the attraction between protons and electrons pulls the atom in tighter.

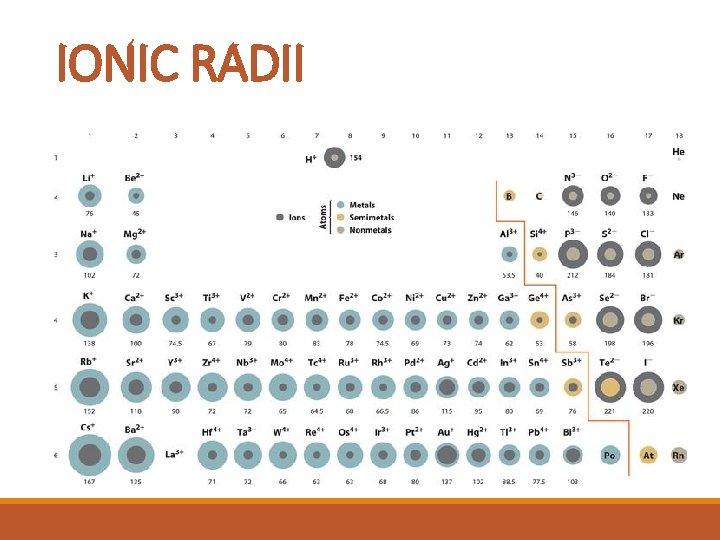
IONIC RADII § The size of an atom’s ion, measured by the radius. § When atoms form a compound, the compound is more stable than the uncombined atoms were. § The outer energy levels are full (like the noble gases).

IONIC RADII

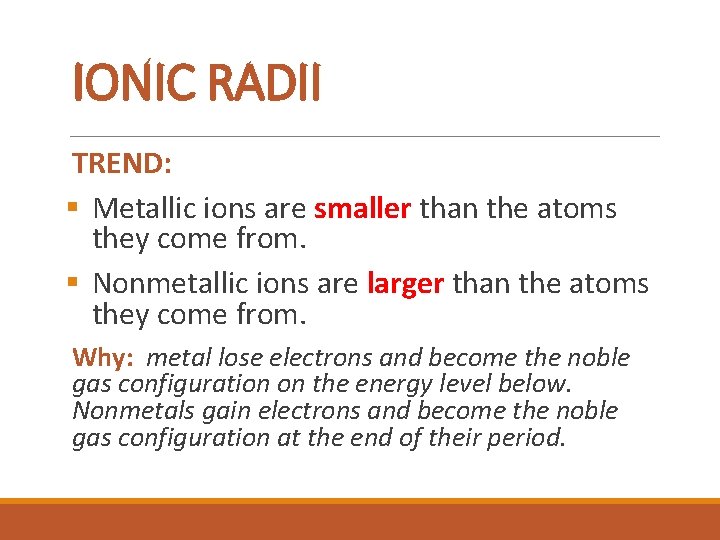
IONIC RADII TREND: § Metallic ions are smaller than the atoms they come from. § Nonmetallic ions are larger than the atoms they come from. Why: metal lose electrons and become the noble gas configuration on the energy level below. Nonmetals gain electrons and become the noble gas configuration at the end of their period.

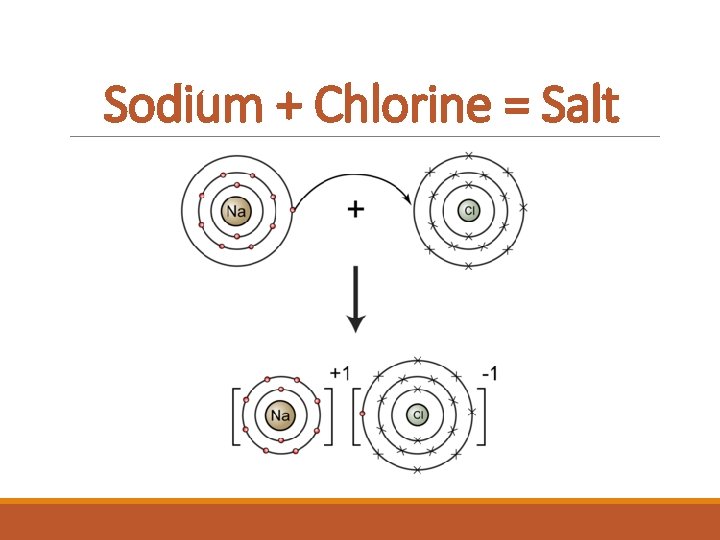
Sodium + Chlorine = Salt

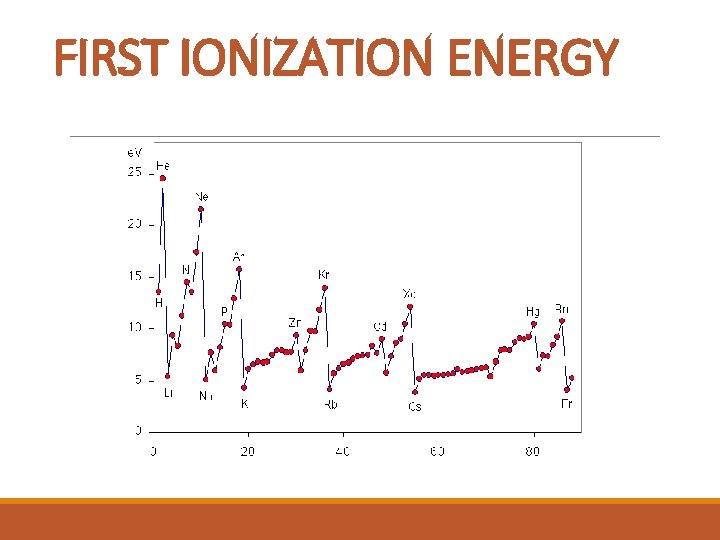
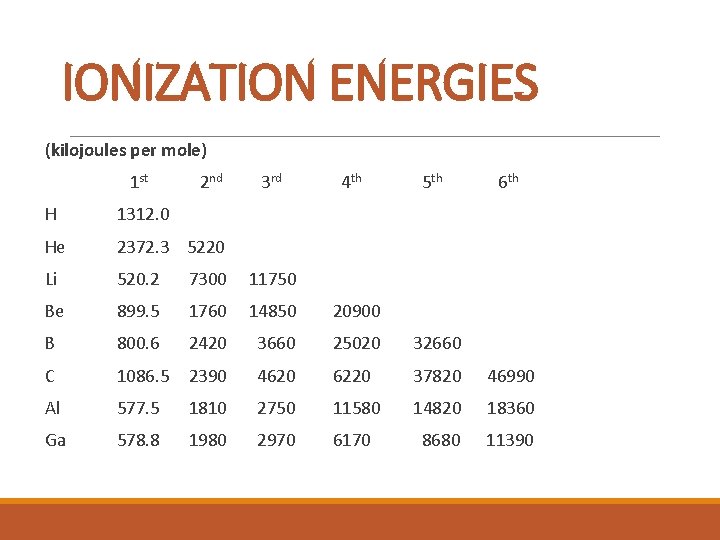
IONIZATION ENERGY § This is the energy required to remove an electron. § The first ionization energy is the energy required to remove the most loosely held electron.

FIRST IONIZATION ENERGY

IONIZATION ENERGY TREND: Ionization energy increases as atomic number increases across a period. In a group, there is a gradual decrease in the first ionization energy as the atomic number increases. Metals have low first ionization energies and nonmetals have high first ionization energies.

IONIZATION ENERGY Why? Across a period, the larger the nuclear charge, the greater the ionization energy; Down a group, the greater shielding effect leads to less ionization energy; With a bigger electron cloud (more energy levels), the ionization energy decreases; an electron in a full or half-full sublevel requires additional energy to be removed.

IONIZATION ENERGIES (kilojoules per mole) 1 st 2 nd 3 rd 4 th 5 th 6 th H 1312. 0 He 2372. 3 5220 Li 520. 2 7300 11750 Be 899. 5 1760 14850 20900 B 800. 6 2420 3660 25020 32660 C 1086. 5 2390 4620 6220 37820 46990 Al 577. 5 1810 2750 11580 14820 18360 Ga 578. 8 1980 2970 6170 8680 11390

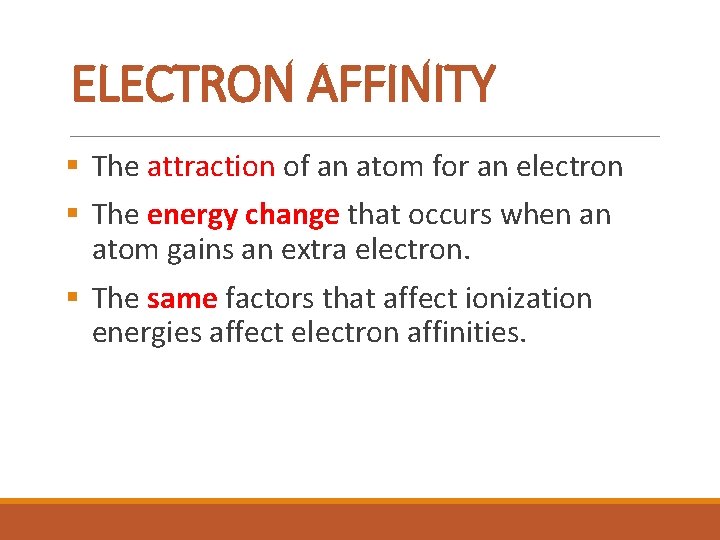
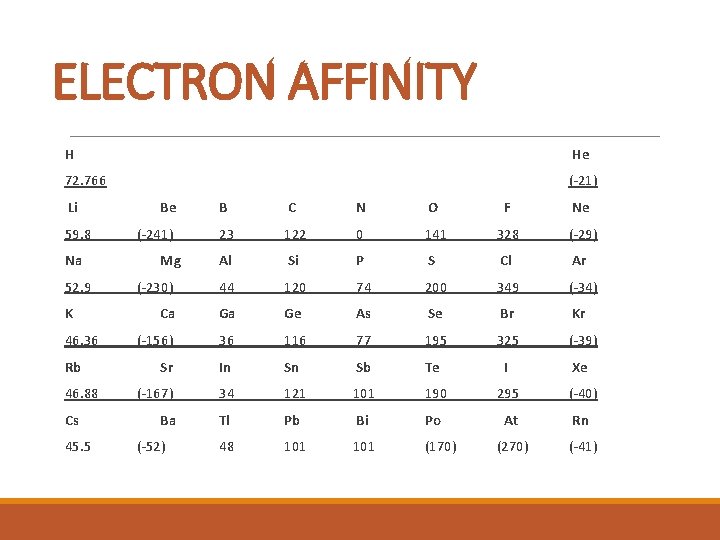
ELECTRON AFFINITY § The attraction of an atom for an electron § The energy change that occurs when an atom gains an extra electron. § The same factors that affect ionization energies affect electron affinities.

ELECTRON AFFINITY H He 72. 766 (-21) Li 59. 8 Na 52. 9 K 46. 36 Rb 46. 88 Cs 45. 5 Be B C N O F Ne 23 122 0 141 328 (-29) Al Si P S Cl Ar (-230) 44 120 74 200 349 (-34) Ca Ga Ge As Se Br Kr (-156) 36 116 77 195 325 (-39) Sr In Sn Sb Te I Xe (-167) 34 121 101 190 295 (-40) Ba Tl Pb Bi Po At Rn 48 101 (170) (270) (-41) (-241) Mg (-52)

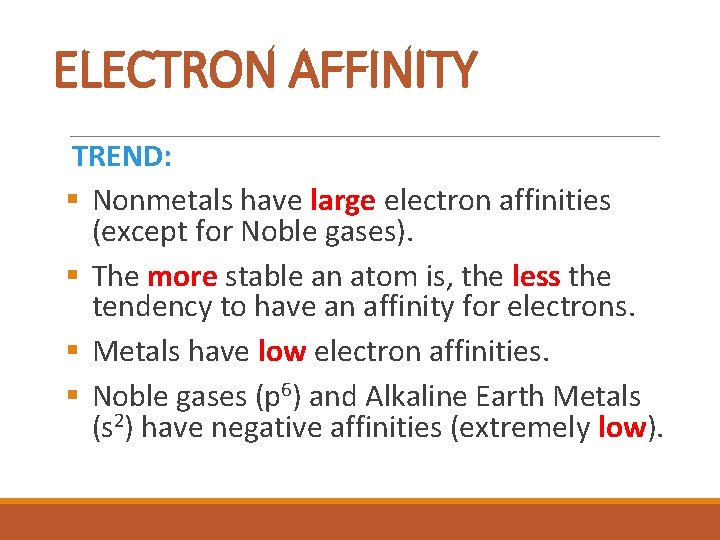
ELECTRON AFFINITY TREND: § Nonmetals have large electron affinities (except for Noble gases). § The more stable an atom is, the less the tendency to have an affinity for electrons. § Metals have low electron affinities. § Noble gases (p 6) and Alkaline Earth Metals (s 2) have negative affinities (extremely low).

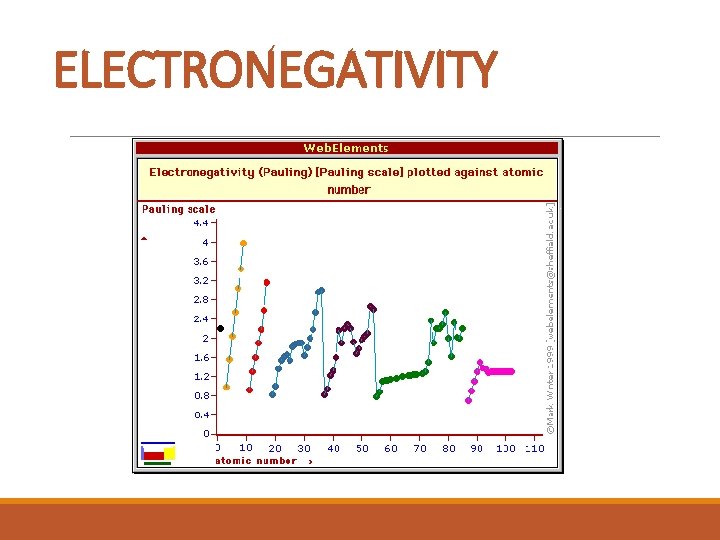
ELECTRONEGATIVITY § A tug of war between atoms for electrons in a chemical bond. § How well the electrons “tug” is electronegativity. § The better the atom ‘tugs’ an electron from another element, the higher the electronegativity § Fluorine is the most electronegative element.

ELECTRONEGATIVITY

ELECTRONEGATIVITY TREND: § increases from left to right across a period as the number of valence electrons increases and the size of the atom decreases. § A low ionization energy and a low electron affinity means low electronegativity.

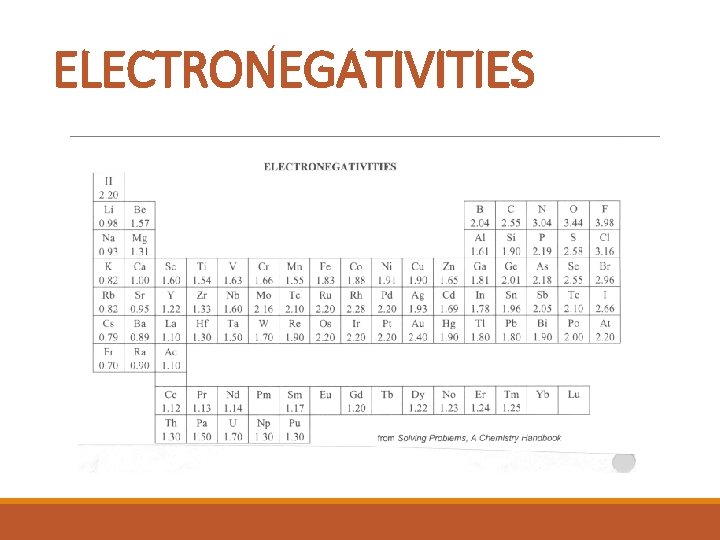
ELECTRONEGATIVITIES

SUMMARY Alkali Metals: - valence electrons s 1 - ionization energy LOW - Electron Affinity LOW - Electronegativity LOW

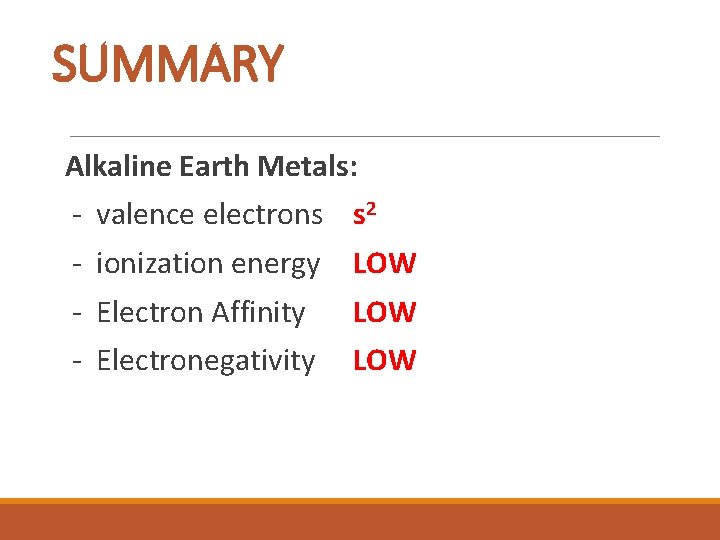
SUMMARY Alkaline Earth Metals: - valence electrons s 2 - ionization energy LOW - Electron Affinity LOW - Electronegativity LOW

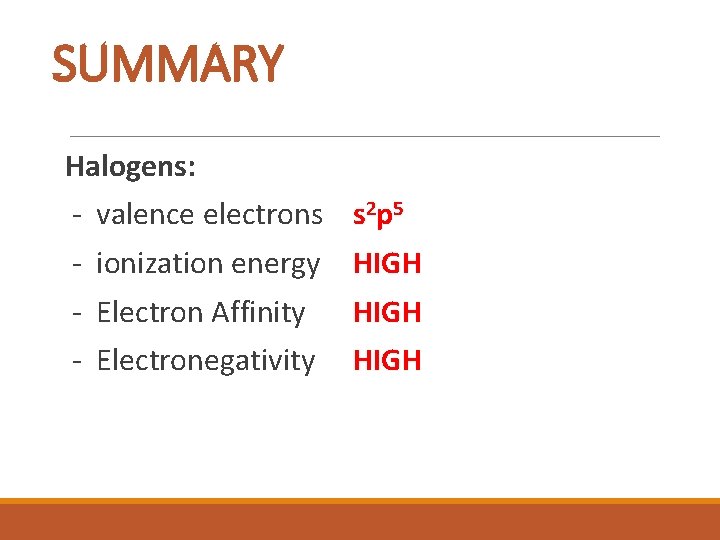
SUMMARY Halogens: - valence electrons s 2 p 5 - ionization energy HIGH - Electron Affinity HIGH - Electronegativity HIGH

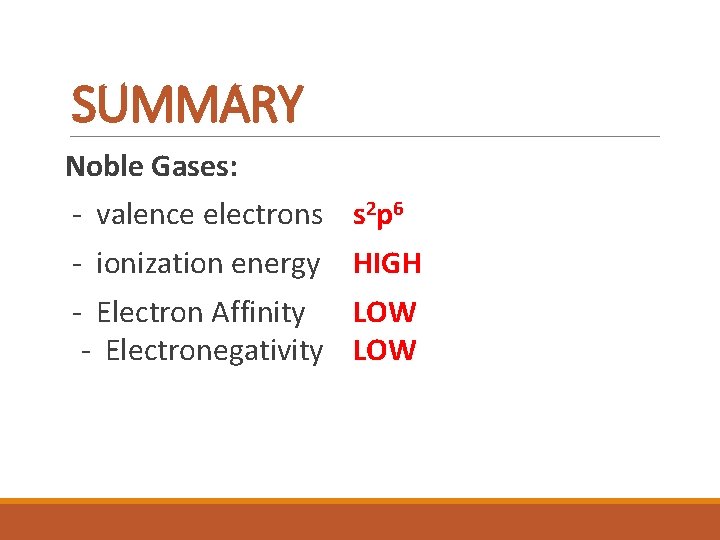
SUMMARY Noble Gases: - valence electrons s 2 p 6 - ionization energy HIGH - Electron Affinity LOW - Electronegativity LOW

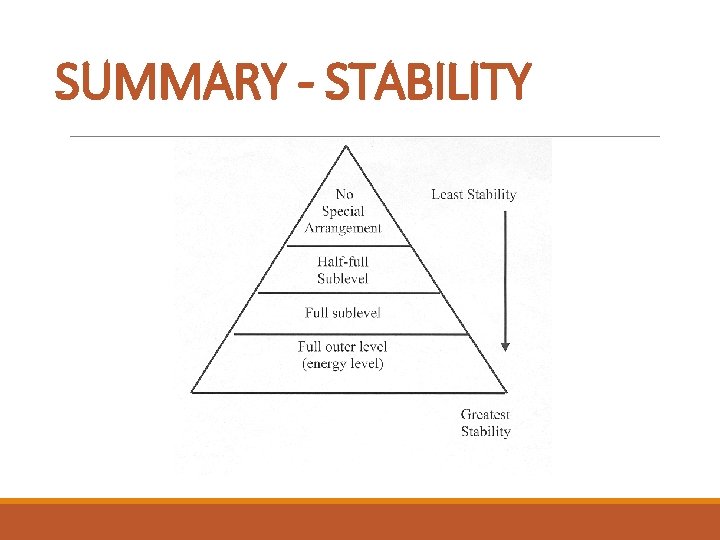
SUMMARY - STABILITY

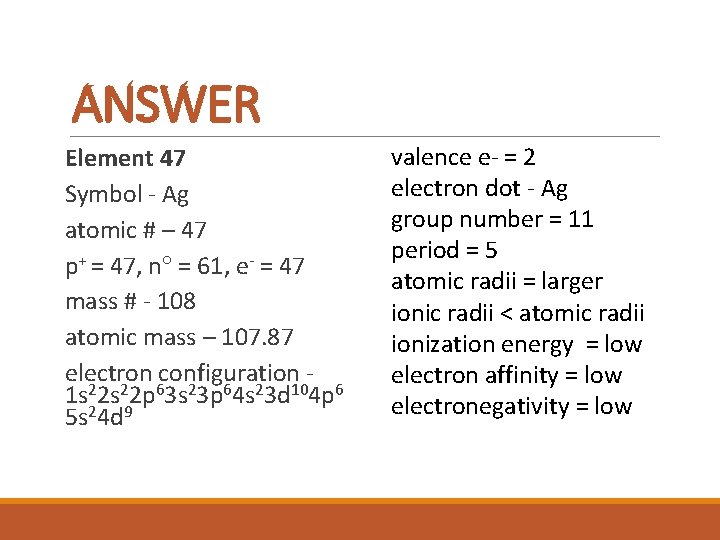
DO NOW For element #47, determine the following info for it using only the periodic table: symbol, atomic number, p+, n , e-, mass #, atomic mass, electron configuration, valence e-, electron dot, group number, period, and atomic radii compared to iodine.

ANSWER Element 47 Symbol - Ag atomic # – 47 p+ = 47, n = 61, e- = 47 mass # - 108 atomic mass – 107. 87 electron configuration 1 s 22 p 63 s 23 p 64 s 23 d 104 p 6 5 s 24 d 9 valence e- = 2 electron dot - Ag group number = 11 period = 5 atomic radii = larger ionic radii < atomic radii ionization energy = low electron affinity = low electronegativity = low

TO DO Backside of notes – due tomorrow