Distillation So simple and yet so complex and











- Slides: 50

Distillation: So simple and yet so complex. . . and vice versa Sigurd Skogestad Norwegian University of Science and Technology (NTNU) Trondheim, Norway

Outline n n n When use distillation Increase in heat input decreases temperature? ? Complex model but simple dynamics. . . . usually Control: Get rid of some myths! Complex column configurations (Petlyuk/Kaibel). . . save energy as well as capital

BASF Aktiengesellschaft

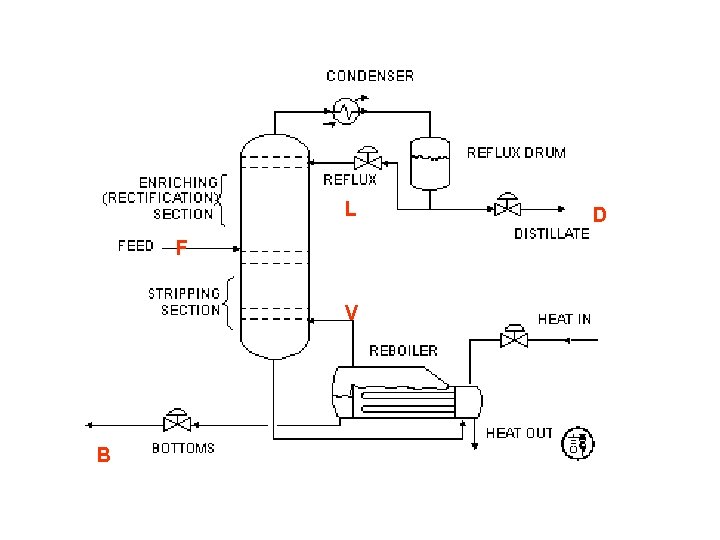
L F V B D

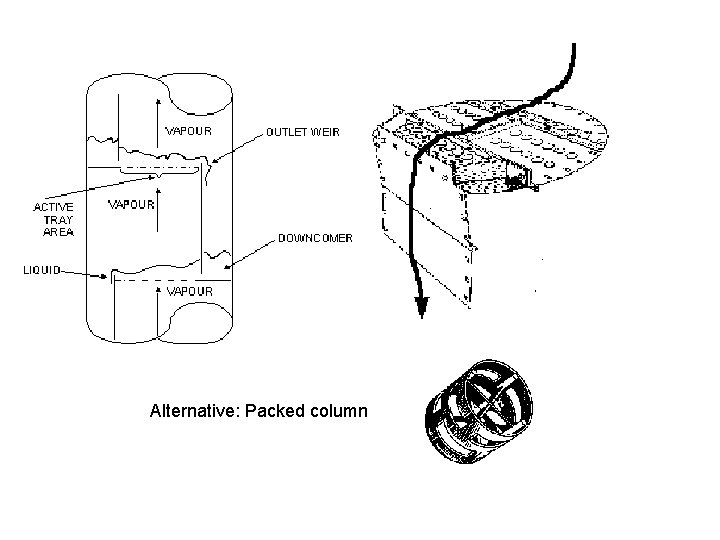
Alternative: Packed column

When use distillation? n n Liquid mixtures with difference in boiling point Unbeatable for high-purity separations because n Essentially same energy usage independent of (im)purity! § n Number of stages increases only as log of impurity! § § n Going from 1% to 0. 0001% (1 ppm) impurity in one product increases required number of stages by factor 3 log(1. e-6)/log(1. e-2)=3 Well suited for scale-up § n Going from 1% to 0. 0001% (1 ppm) impurity in one product increases energy usage only by about 1% Columns with diameters over 15 m Examples of unlikely uses of distillation: § § High-purity silicon for computers (via Si. Cl 3 distillation) Water – heavy-water separation (boiling point difference only 1. 4 C)

Reflux gives strange effects

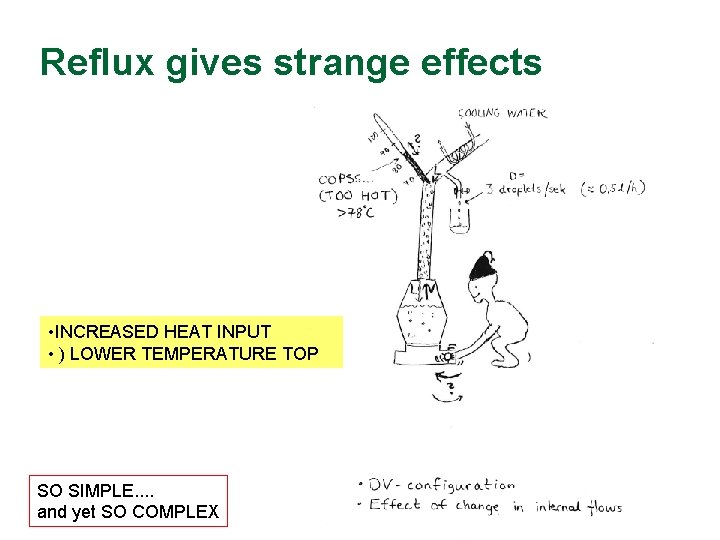
Reflux gives strange effects • INCREASED HEAT INPUT • ) LOWER TEMPERATURE TOP SO SIMPLE. . and yet SO COMPLEX

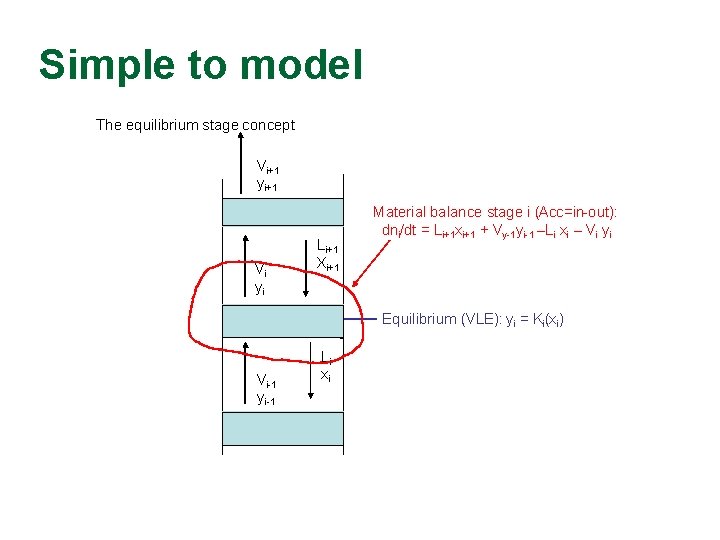
Simple to model The equilibrium stage concept Vi+1 yi+1 Stage i+1 Vi yi Li+1 Xi+1 Equilibrium (VLE): yi = Ki(xi) Stage i Vi-1 yi-1 Material balance stage i (Acc=in-out): dni/dt = Li+1 xi+1 + Vy-1 yi-1 –Li xi – Vi yi Li xi Stage i-1 The equlibrium stage concept is used for both tray and packed columns • N = no. of equilibrium stages in column Typical: 0. 7 • Tray column: N = No. trays * Tray-efficiency Typical: 0. 5 m • Packed columns: N = Height [m] / HETP [m]

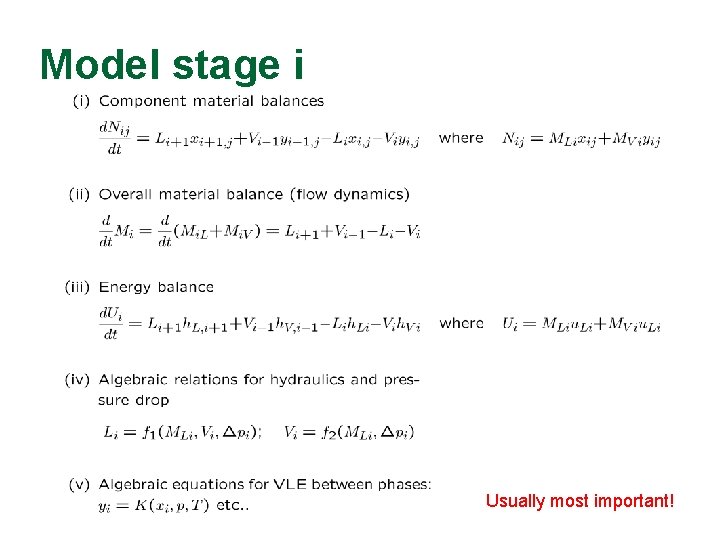
Model stage i Usually most important!

Simple to model. . . yet difficult to understand n SIMPLE TO MODEL q q n 1920’s: Models known. Graphical solution (Mc. Cabe-Thiele) 1960’s: Simulation with computer straightforward No need for more work!? BEHAVIOR NOT SO SIMPLE TO UNDERSTAND q Mathematician: n n n q Simulation and experience n n q Large number of coupled equations Nonlinear equations (mainly VLE) Complex behavior expected Not so complex Dynamic response: simple More simulations: Maybe not so simple after all n n Instability Multiple steady states

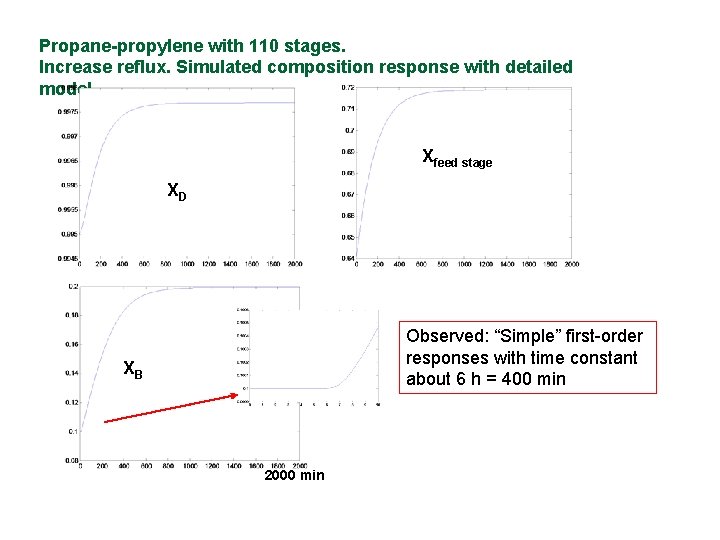
Dynamic behavior simple! n Example: Composition response of propanepropylene splitter with 110 stages and large reflux

Propane-propylene with 110 stages. Increase reflux. Simulated composition response with detailed model. Xfeed stage XD Observed: “Simple” first-order responses with time constant about 6 h = 400 min XB 2000 min

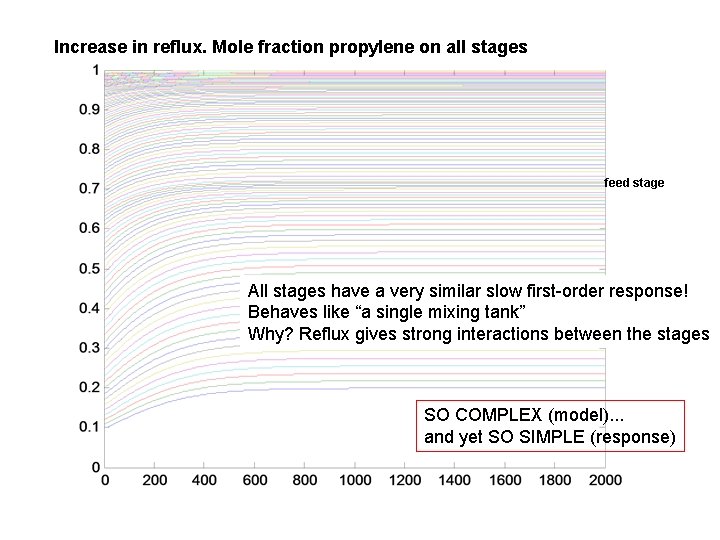
Increase in reflux. Mole fraction propylene on all stages feed stage All stages have a very similar slow first-order response! Behaves like “a single mixing tank” Why? Reflux gives strong interactions between the stages SO COMPLEX (model). . . and yet SO SIMPLE (response)

Dynamic behavior simple? n 1970’s and 1980’s: Mathematical proofs that dynamics are always stable q n In reality, independent variables are q q n Based on analyzing dynamic model with L and V [mol/s] as independent variables Lw [kg/s] = L [mol/s] ¢ M [kg/mol] QB [J/s] = V [mol/s] ¢ Hvap [J/mol] Does it make a difference? YES, in some cases!

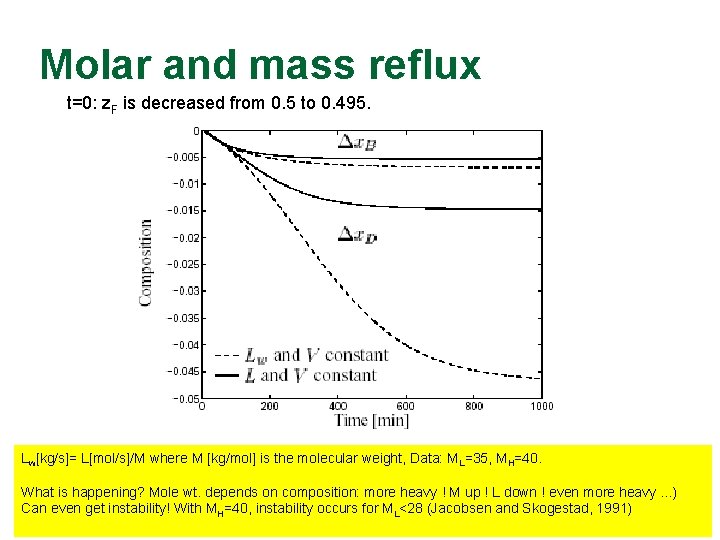
Molar and mass reflux t=0: z. F is decreased from 0. 5 to 0. 495. Lw[kg/s]= L[mol/s]/M where M [kg/mol] is the molecular weight, Data: ML=35, MH=40. What is happening? Mole wt. depends on composition: more heavy ! M up ! L down ! even more heavy. . . ) Can even get instability! With MH=40, instability occurs for ML<28 (Jacobsen and Skogestad, 1991)

Instability for “ideal” columns: Many people didn’t believe us when we first presented it in 1991! Likely to happen if the mole weights are sufficiently different

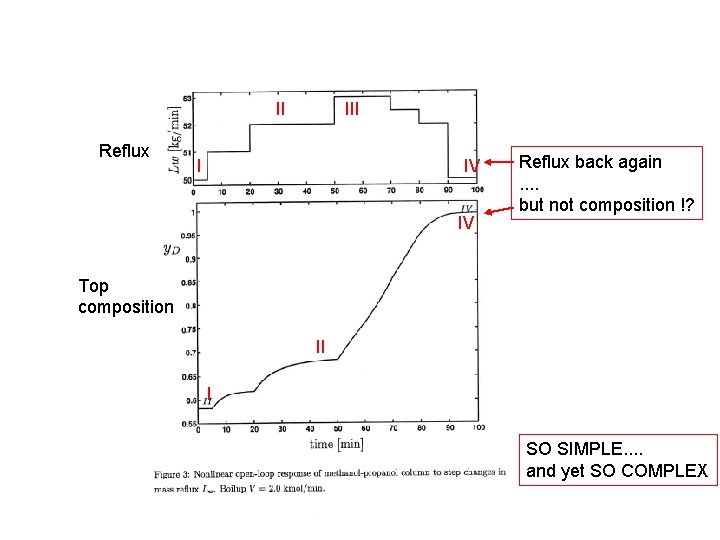
II Reflux III I IV IV Reflux back again. . but not composition !? Top composition II I SO SIMPLE. . and yet SO COMPLEX

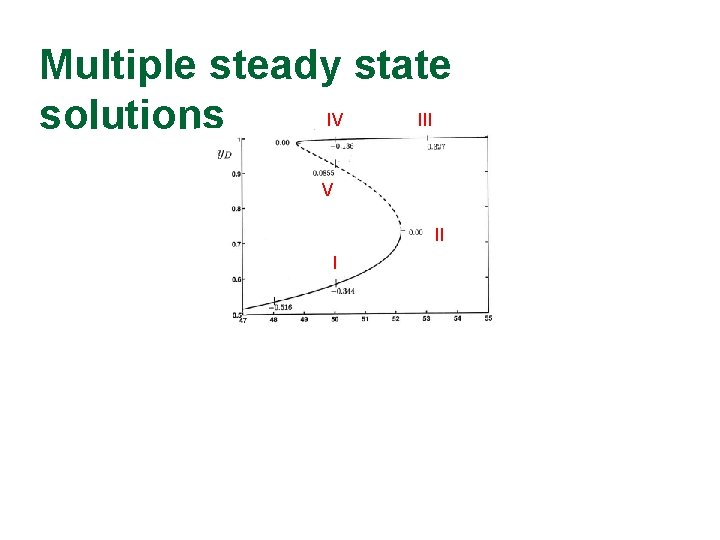
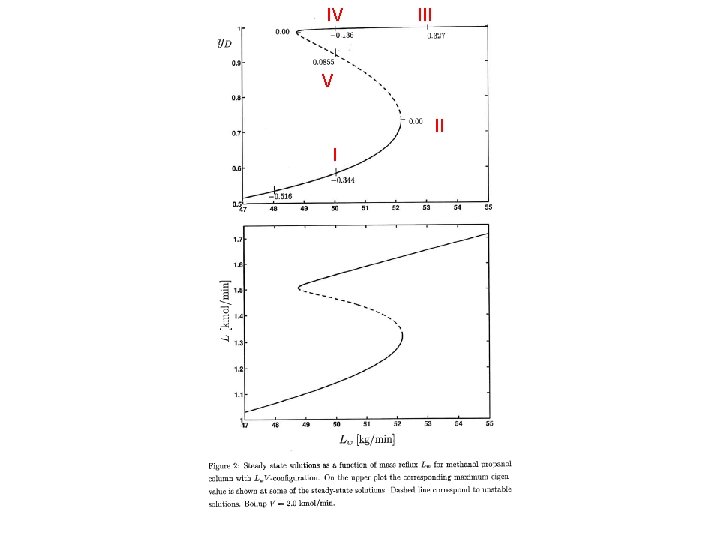
Multiple steady state IV III solutions V II I

IV III V II I

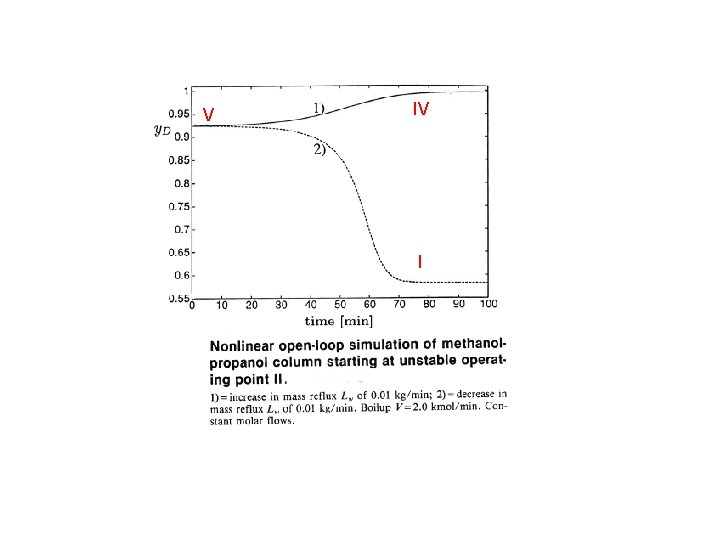
V IV I

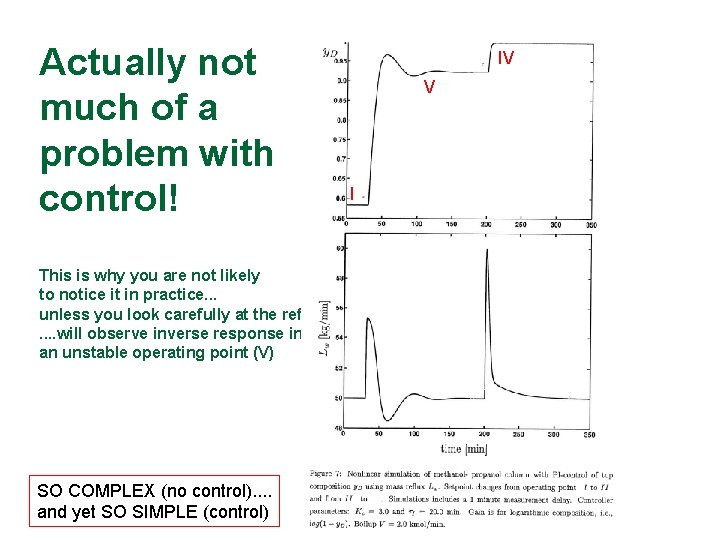
Actually not much of a problem with control! This is why you are not likely to notice it in practice. . . unless you look carefully at the reflux. . will observe inverse response in an unstable operating point (V) SO COMPLEX (no control). . and yet SO SIMPLE (control) IV V I


Myth of slow control n n Let us get rid of it!!! Compare manual (“perfect operator”) and automatic control for typical column: n n n q q 40 stages, Binary mixture with 99% purity both ends, relative volatility = 1. 5 First “one-point” control: Control of top composition only Then “two-point” control: Control of both compositions

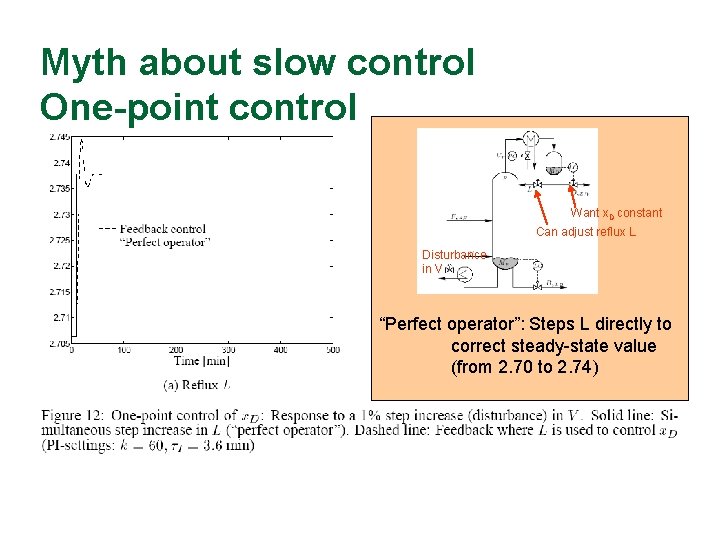
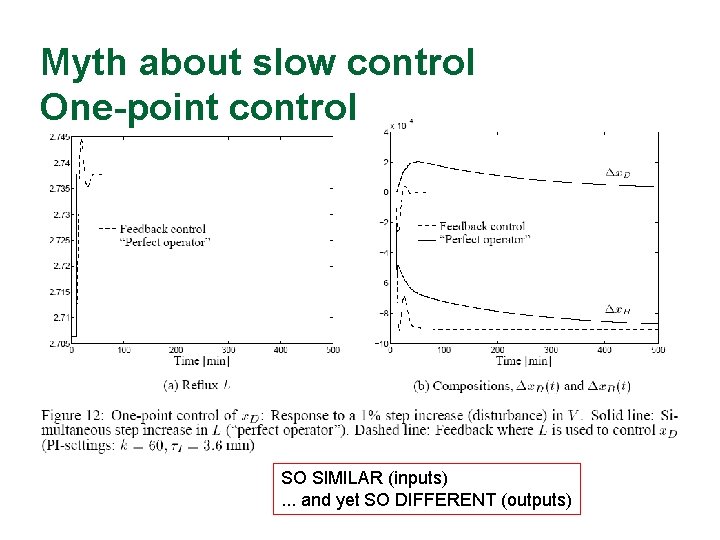
Myth about slow control One-point control Want x. D constant Can adjust reflux L Disturbance in V “Perfect operator”: Steps L directly to correct steady-state value (from 2. 70 to 2. 74)

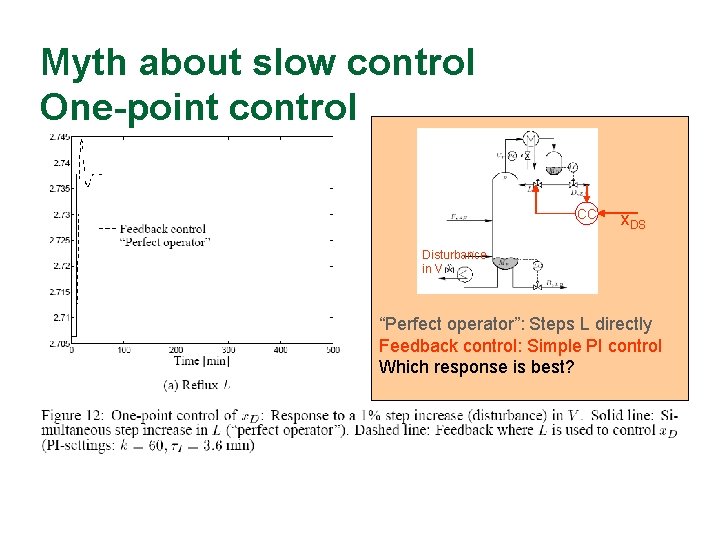
Myth about slow control One-point control CC x. DS Disturbance in V “Perfect operator”: Steps L directly Feedback control: Simple PI control Which response is best?

Myth about slow control One-point control SO SIMILAR (inputs). . . and yet SO DIFFERENT (outputs)

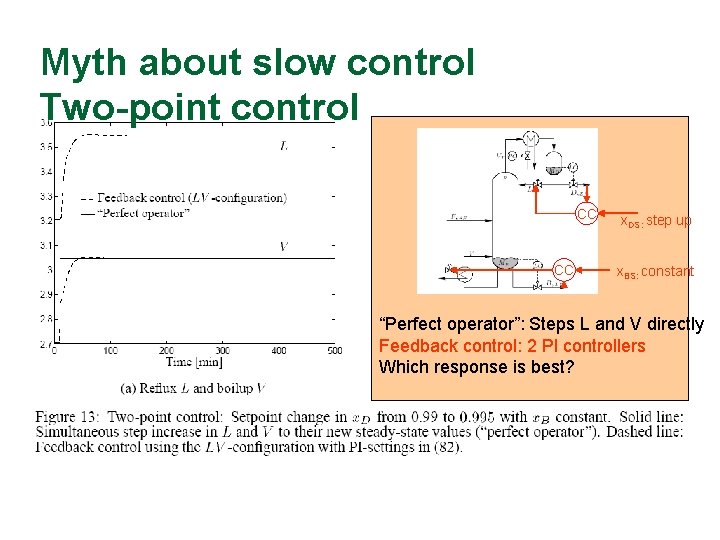
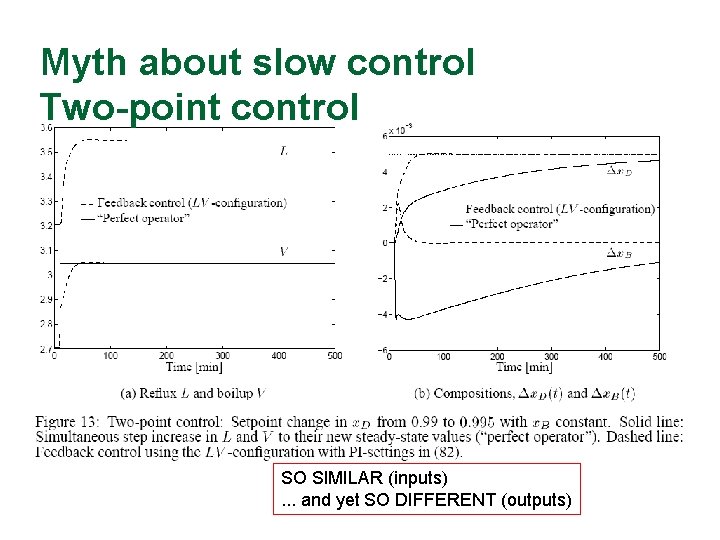
Myth about slow control Two-point control CC CC x. DS: step up x. BS: constant “Perfect operator”: Steps L and V directly Feedback control: 2 PI controllers Which response is best?

Myth about slow control Two-point control SO SIMILAR (inputs). . . and yet SO DIFFERENT (outputs)

Myth about slow control Conclusion: n Experience operator: Fast control impossible q n BUT, with feedback control the response can be fast! q q n “takes hours or days before the columns settles” Feedback changes the dynamics (eigenvalues) Requires continuous “active” control Most columns have a single slow mode (without control) q Sufficient to close a single loop (typical on temperature) to change the dynamics for the entire column

Complex columns n Sequence of columns for multicomponent separation Heat integration Pressure levels Integrated solutions Non-ideal mixtures (azeotropes) n Here: Will consider “Petlyuk” columns n n

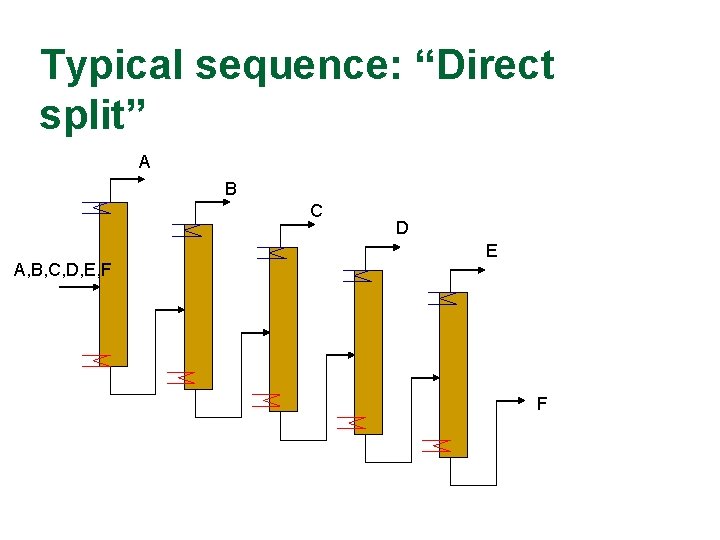
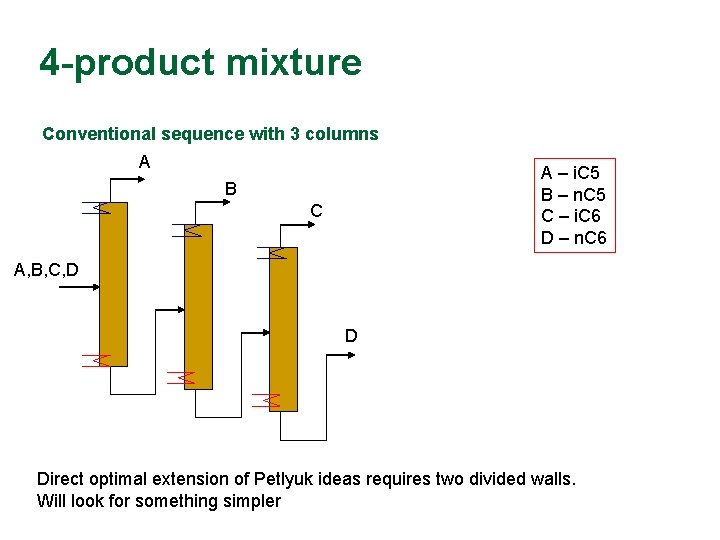
Typical sequence: “Direct split” A B C A, B, C, D, E, F D E F

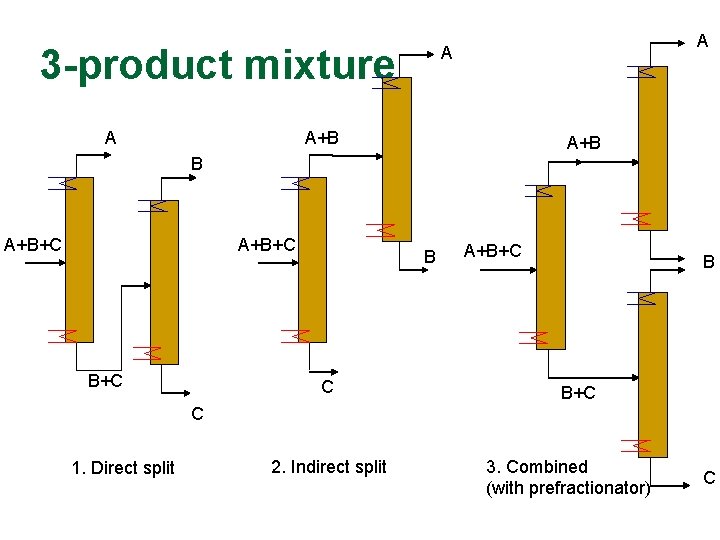
3 -product mixture A A+B A+B+C B+C B C A+B+C B B+C C 1. Direct split 2. Indirect split 3. Combined (with prefractionator) C

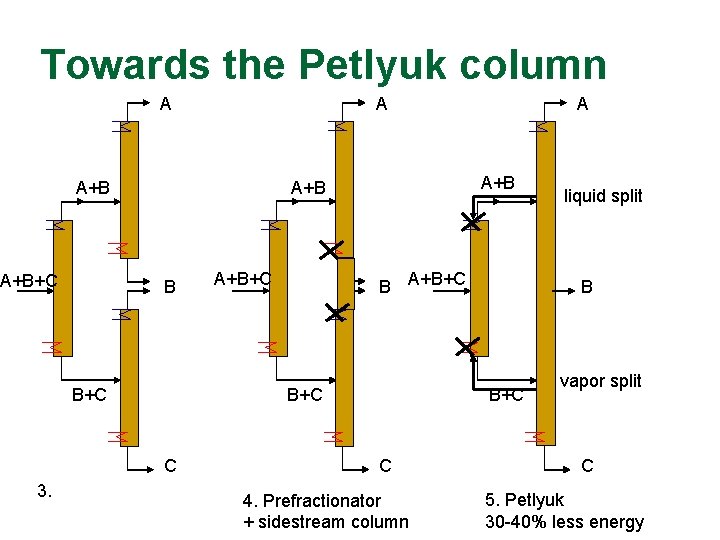
Towards the Petlyuk column A A A+B+C B+C C 3. A+B A+B A B B+C C 4. Prefractionator + sidestream column liquid split vapor split C 5. Petlyuk 30 -40% less energy

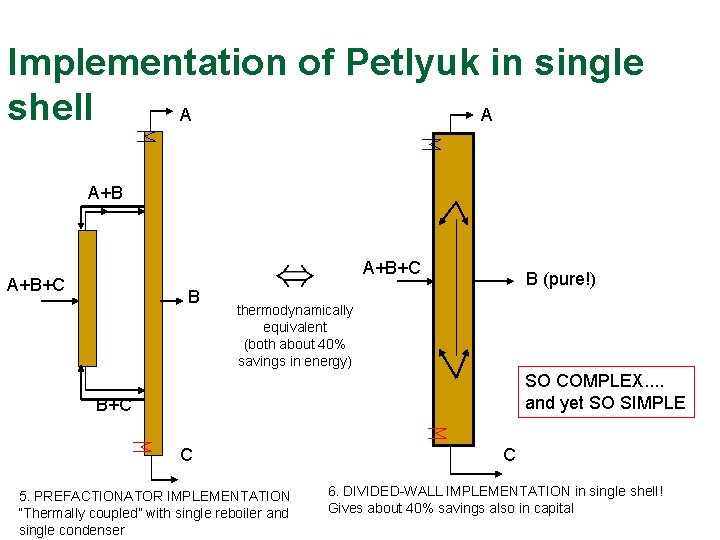
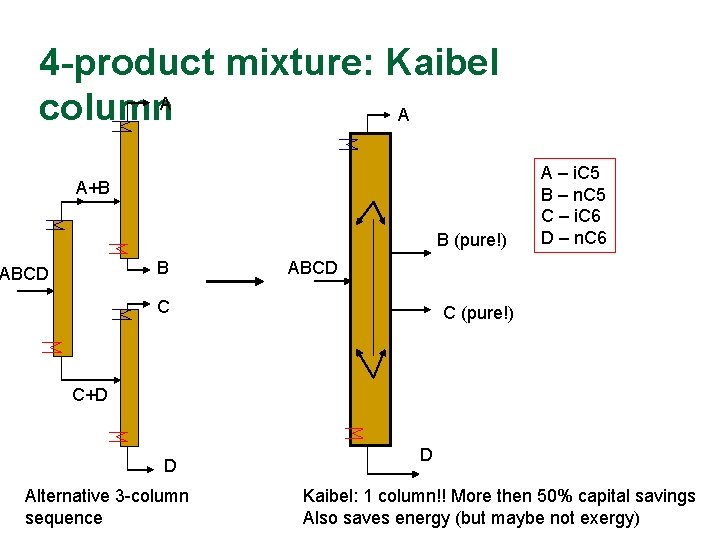
Implementation of Petlyuk in single shell A A A+B+C B B (pure!) thermodynamically equivalent (both about 40% savings in energy) SO COMPLEX. . and yet SO SIMPLE B+C C 5. PREFACTIONATOR IMPLEMENTATION “Thermally coupled” with single reboiler and single condenser C 6. DIVIDED-WALL IMPLEMENTATION in single shell! Gives about 40% savings also in capital

GC – Chemicals Research and Engineering Dividing Wall Columns Off-center Position of the Dividing Wall ≈ Montz

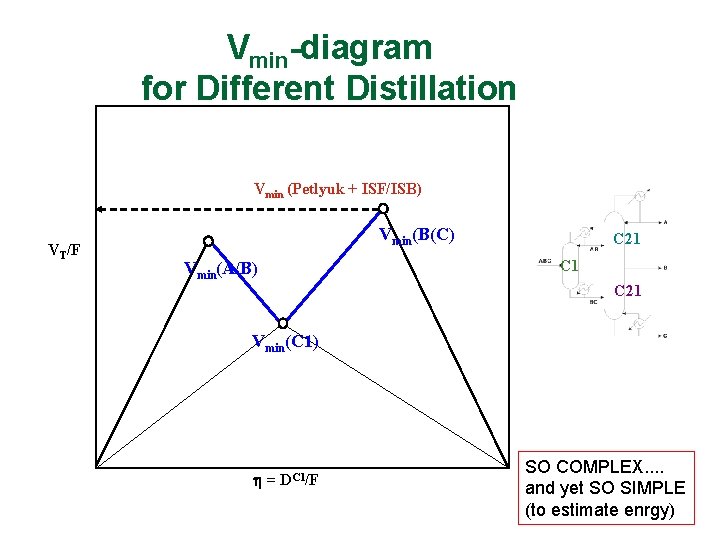
Vmin-diagram for Different Distillation Arrangements PB/C Vmin (Petlyuk + ISF/ISB) PA/B VT/F Vmin(B(C) Vmin(A/B) C 21 C 1 PA/C C 21 Vmin(C 1) = DC 1/F SO COMPLEX. . and yet SO SIMPLE (to estimate enrgy)

Divided wall columns: starting to catch on n n 1940’s: first patent 1960’s: Thermodynamic analysis (Petlyuk) 1984: First implementation (BASF) 2005: BASF has about 50 divided wall columns q n also in Japan, South Africa. . . Control issues still not quite solved q but I think it should be rather easy

4 -product mixture Conventional sequence with 3 columns A A – i. C 5 B – n. C 5 C – i. C 6 D – n. C 6 B C A, B, C, D D Direct optimal extension of Petlyuk ideas requires two divided walls. Will look for something simpler

4 -product mixture: Kaibel column. A A A+B B (pure!) B ABCD A – i. C 5 B – n. C 5 C – i. C 6 D – n. C 6 ABCD C C (pure!) C+D D Alternative 3 -column sequence D Kaibel: 1 column!! More then 50% capital savings Also saves energy (but maybe not exergy)

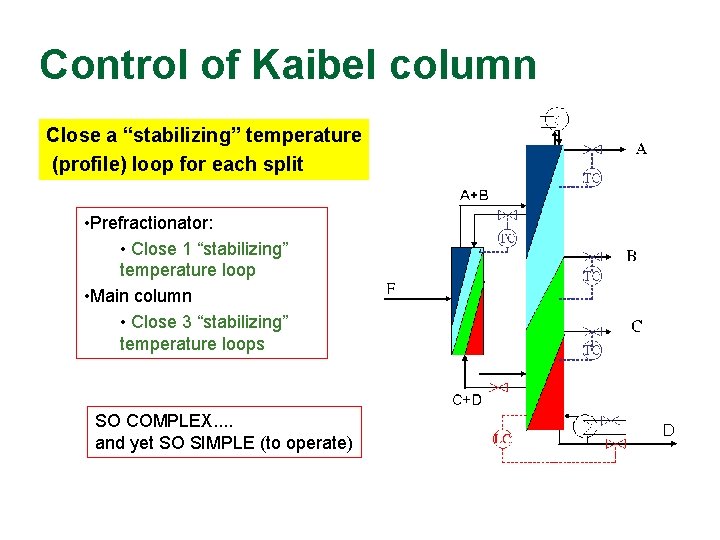
Control of Kaibel column Close a “stabilizing” temperature (profile) loop for each split • Prefractionator: • Close 1 “stabilizing” temperature loop • Main column • Close 3 “stabilizing” temperature loops SO COMPLEX. . and yet SO SIMPLE (to operate) D

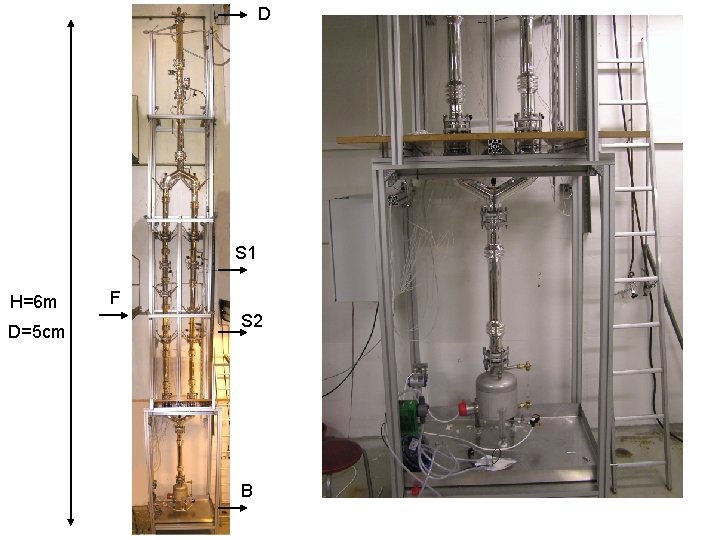
D S 1 H=6 m D=5 cm F S 2 B

Conclusion n n Distillation is important Distillation is unbeatable (in some cases) Distillation is fun Distillation is complex yet simple. . . and vice versa

n n n n column, uses, when use? strange responses. . . increase heat. . T drops model complex: would expect complicated behavior. . . yet simple: show typical response e. g all stages response simple: expect always stable. . . yet complex: can be unstable with mss (Lw V) NEW column configurations. . . “easy first” Petlyuk. Kaibel. make drawing of how it evolves Better. heat-integrated Petlyuk (prefrac). Hidic item BATCH DISTILLATION (Reflux) item MODEL, DYNAMICS (Feedback) item CONTROL (Steady-state misleading) item MULTIPLE STEADY STATES AND INSTABILITY (Nonlinearity and feedback) item INTERLINKED COLUMNS (Parallel paths) item BATCH DISTILLATION AGAIN item SYSTEMS VIEW item CONCLUSION

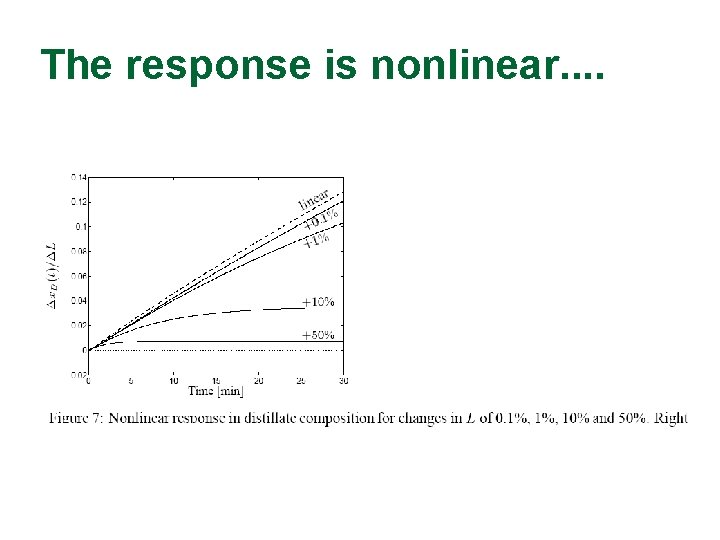
The response is nonlinear. .

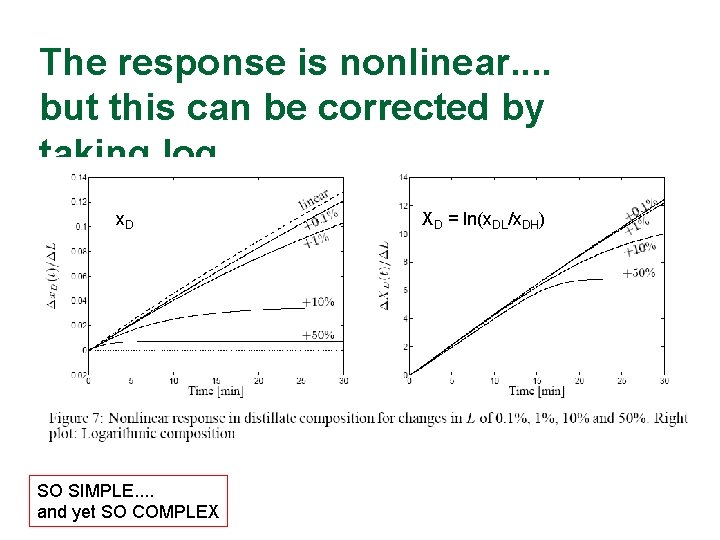
The response is nonlinear. . but this can be corrected by taking log x. D SO SIMPLE. . and yet SO COMPLEX XD = ln(x. DL/x. DH)

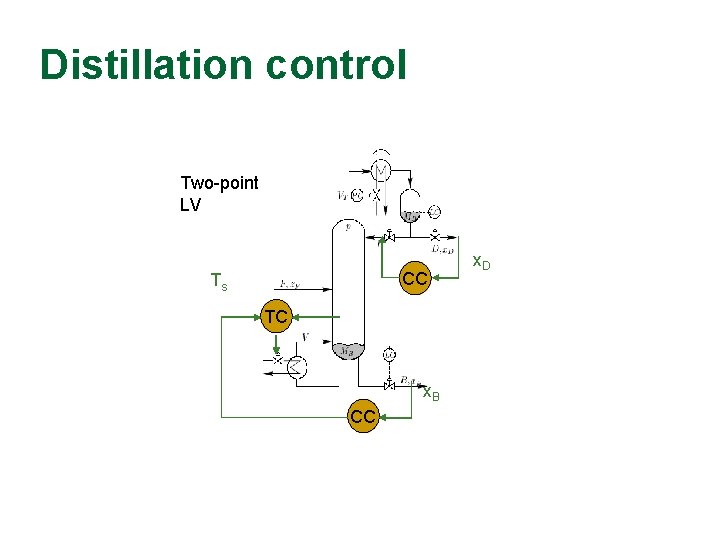
Distillation control Two-point LV LV CC Ts TC x. B CC x. D

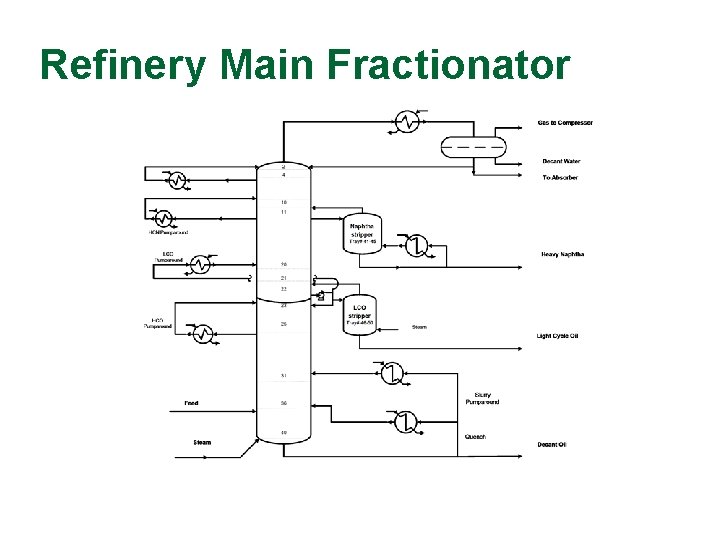
Refinery Main Fractionator

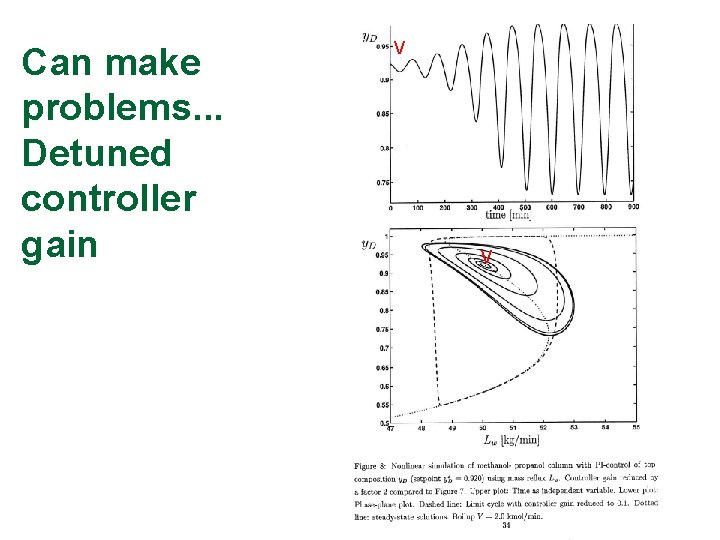
Can make problems. . . Detuned controller gain V V

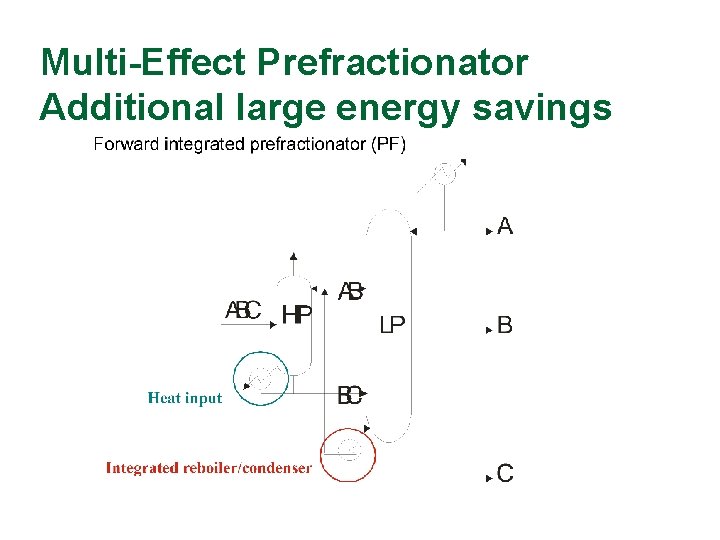
Multi-Effect Prefractionator Additional large energy savings