Understanding distillation Aseel Samaro Introduction Distillation is used

















- Slides: 18

Understanding distillation Aseel Samaro



Introduction § Distillation is used in making perfumes, fuels (such as petrol) and alcoholic drinks (such as vodka). § It is an important separation process involving heating and cooling.

Heating and cooling § On a cold day water vapour from a bath or kettle can condense on a cold surface. § It cools down and turns back to water. § This is what happens in distillation. § Liquid mixtures can be separated using distillation.

Name three substances that are made using distillation. Why does steam turn into liquid water when it touches a window?


Catching steam § When water boils it is hard to catch all of the water vapour because it mixes into the air. § In distillation the vapour is cooled, which allows it to be collected as a liquid. § 2000 years ago Greek scientists, known as alchemists, invented a way to distil liquids. § It was so successful that the design was on sale until 1860.

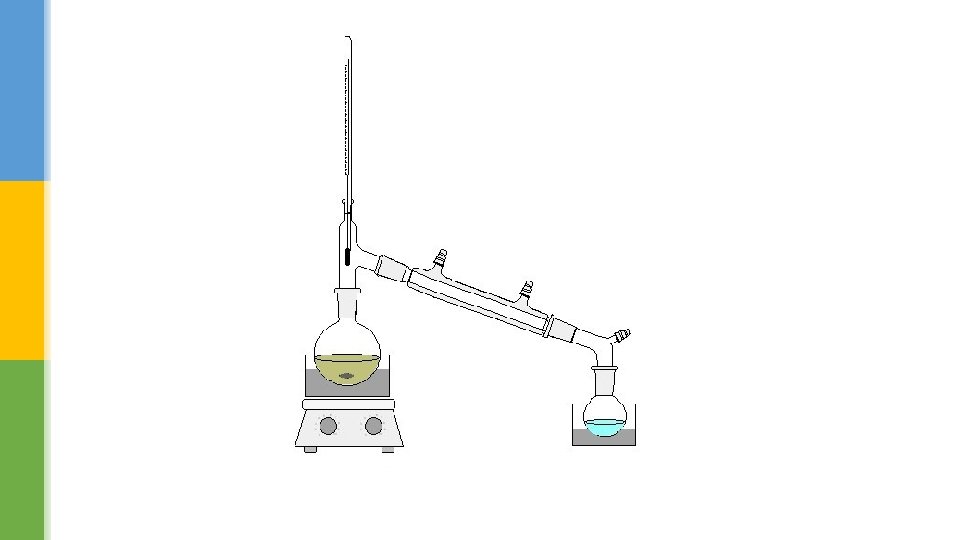
§ The distillation apparatus that we use today is based on the same principle of heating and cooling. § The major improvement is the Liebig condenser, which is a double glass tube. § The hot vapour from the boiling liquid flows through the inner tube, while cold water runs though the outer tube. § This keeps the inner glass tube cold and condenses most vapours easily. The liquid collected at the end is called the distillate.


Why is the alchemists’ method a better method of separation than just heating a mixture of liquids? It stops the steam/vapour/water escaping; captures the steam/vapour/gas and turns it back/ condenses it to water/liquid. Why is the Liebig condenser better than the alchemists’ equipment? The condenser has water to keep the tube cold; which makes condensation happen more quickly; because it is colder. Explain the safety checks you would use to separate a mixture safely. clamp flask above heat source glass not cracked/broken not too much liquid in distillation flask to boil over cold water for condenser is on and does not leak collection beaker is in the right place/no spills.

Distilling mixture § There are two changes of state in distillation. § First, a liquid is evaporated by heating and then the cooled vapour condensed back to a liquid. § When salty water is heated, only the water (solvent) changes state and the salt (solute) is left behind. § The water produced is called distilled water.

§ Different liquids boil at different temperatures – for example, ethanol boils at 78 °C and water at 100 °C. § This means that mixtures of liquids can be separated using distillation. § A thermometer at the top of a distillation flask shows the temperature of the vapour being condensed and hence identifies the substance being separated. § Distillation is an effective way of purifying alcohol or increasing the concentration of alcoholic drinks. § It is also useful for separating flammable liquids like petrol and diesel because the vapours never come into direct contact with the flame.



Why is distillation a better way to separate salt and water than crystallisation? It is quicker collects the water and the salt less water/salt is lost. Why is a thermometer important in distillation? The thermometer gives the temperature of the vapour/boiling point so you know which substance is being separated – i. e. which is a gas (in the condenser) and which is still liquid (in the flask). Why is distillation a good method for separating petrol and diesel? They are flammable liquids; a direct flame cannot be used. Explain how water and ethanol are separated. They have different boiling points/ethanol has a lower boiling point/turns to vapour/gas first; and can be condensed and collected before the water boils.

Did you know ? § Steam distillation is used to obtain essential oils from plants such as herbs and flowers. § The products are used in aromatherapy, flavourings in foods and drinks, and as scents in perfumes, cosmetics and cleaning products.

HOMEWORK
 How to identify pure aseel
How to identify pure aseel Aseel yousef
Aseel yousef Lab samaro
Lab samaro Lab-samaro
Lab-samaro Lab samaro
Lab samaro Samaro property
Samaro property Difference between distillation and fractional distillation
Difference between distillation and fractional distillation Raoult's law vs dalton's law
Raoult's law vs dalton's law Definition of distilation
Definition of distilation Application for distillation
Application for distillation Theory of distillation
Theory of distillation Simple distillation
Simple distillation In a premix burner used in fes the fuel used is
In a premix burner used in fes the fuel used is In a premix burner used in fes the fuel used is
In a premix burner used in fes the fuel used is Introduction paragraph structure
Introduction paragraph structure Rayleigh distillation
Rayleigh distillation Azeotrope distillation
Azeotrope distillation Is maple syrup a pure substance
Is maple syrup a pure substance Mixture separated by distillation
Mixture separated by distillation