Determination of Henrys Law constants of organic compounds








- Slides: 13

Determination of Henry’s Law constants of organic compounds through use of an analytical air stripper with structured packing. REU program, Summer 2000 Julie P. Caires, Washington State University Michiya Suzuki, University of Oklahoma Dr. Tohren Kibbey, University of Oklahoma

Air stripping Water Air containing to be organic vapors out treated Filled with packing material Air in Clean water out • Packed column air stripping – Filled with porous packing material – Increased surface area of water on packing material allows organic compounds to volatilize into moving air – Effectiveness depends on Henry’s Law constant for the organic compound, liquid flow rate, gas flow rate, temperature, size of column, and properties of the packing material.

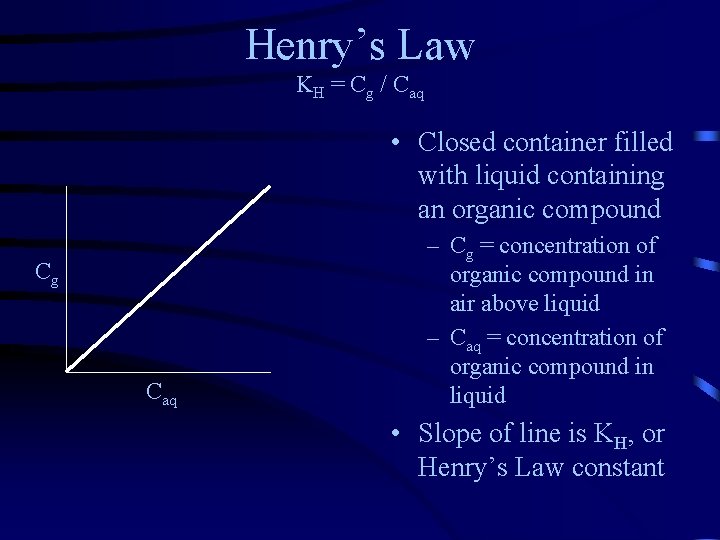
Henry’s Law KH = Cg / Caq • Closed container filled with liquid containing an organic compound Cg Caq – Cg = concentration of organic compound in air above liquid – Caq = concentration of organic compound in liquid • Slope of line is KH, or Henry’s Law constant

The Stripping Factor (S) • S = (G * KH) / L – G = gas flow rate – L = liquid flow rate – KH = Henry’s Law constant

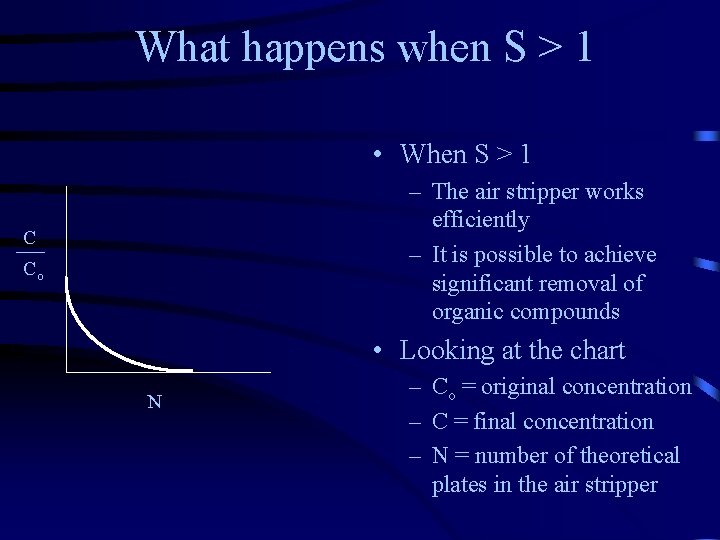
What happens when S > 1 • When S > 1 – The air stripper works efficiently – It is possible to achieve significant removal of organic compounds C Co • Looking at the chart N – Co = original concentration – C = final concentration – N = number of theoretical plates in the air stripper

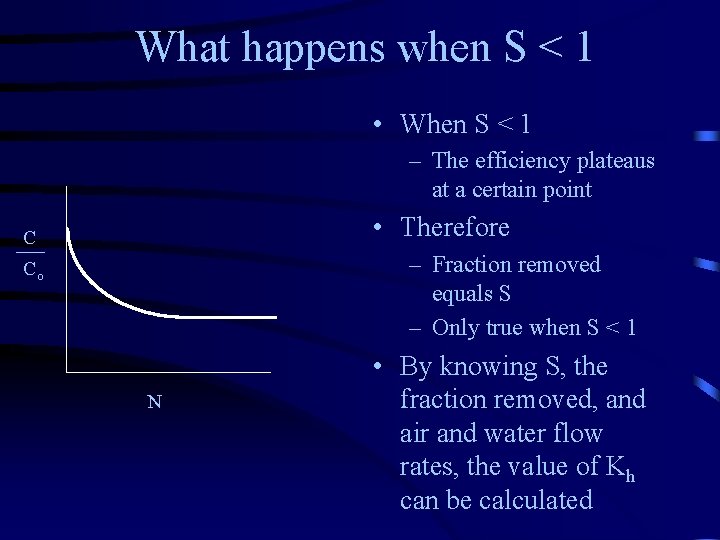
What happens when S < 1 • When S < 1 – The efficiency plateaus at a certain point • Therefore C – Fraction removed equals S – Only true when S < 1 Co N • By knowing S, the fraction removed, and air and water flow rates, the value of Kh can be calculated

Preliminary experiments: • Temperature profiling at different G and L rates along the air stripping column – Large (5. 2 C) temperature drop – Necessary to build an air saturator • Determination of equilibration time – Used red food coloring – Preliminary data: 35 minutes, later extended to 1 hour

Experiments conducted: • Determining Henry’s law constant – Isopropanol (IPA) – Methyl tert-butyl ether (MTBE) • Determining effect of co-solubility on KH – MTBE added to different concentrations of IPA

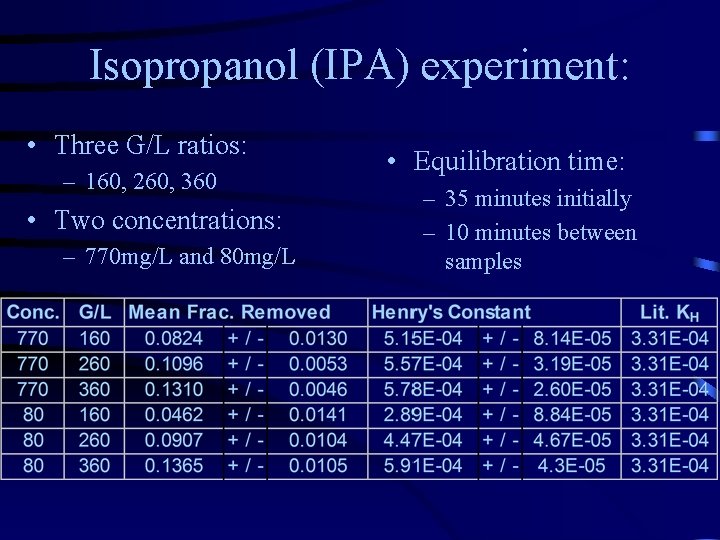
Isopropanol (IPA) experiment: • Three G/L ratios: – 160, 260, 360 • Two concentrations: – 770 mg/L and 80 mg/L • Equilibration time: – 35 minutes initially – 10 minutes between samples

Methyl-tert butyl ether (MTBE): • Run #1: • Run #2: – Concentration: 500 mg/L • Equilibration time: – 35 minutes initially, 10 minutes between samples – 60 minutes initially, 20 minutes between samples • G/L ratios: 10, 20 • G/L ratio: 30

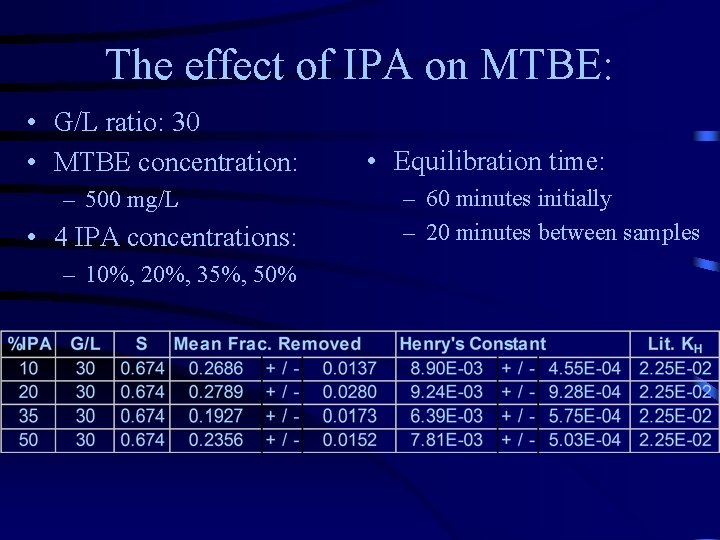
The effect of IPA on MTBE: • G/L ratio: 30 • MTBE concentration: – 500 mg/L • 4 IPA concentrations: – 10%, 20%, 35%, 50% • Equilibration time: – 60 minutes initially – 20 minutes between samples

Conclusions: • Analytical air stripper is effective – With low volatility compounds – With 1 hour initial equilibration time – At higher G/L ratios • Effect of co-solvent (IPA) – Dramatic decrease in Henry’s law constant

Acknowledgments • • • NSF Dr. Kibbey Michiya Suzuki Joe (for the air saturator setup idea) University of Oklahoma
 Henrys law
Henrys law Patrick henrys speech
Patrick henrys speech A small sports car collides head on
A small sports car collides head on Henrys scrabble
Henrys scrabble P=k/v
P=k/v Kc kp
Kc kp Vitamins are tasteless organic compounds
Vitamins are tasteless organic compounds Vitamin classification chart
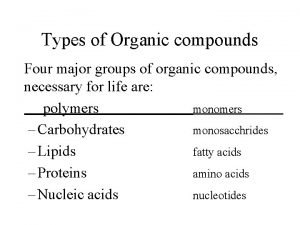
Vitamin classification chart Four types of organic compounds
Four types of organic compounds Decomposition of organic matter equation
Decomposition of organic matter equation Organic and inorganic compounds experiment
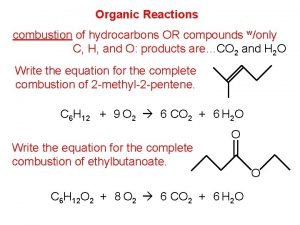
Organic and inorganic compounds experiment Organic combustion
Organic combustion These are organic compounds made by living things
These are organic compounds made by living things All organic compounds must contain the element
All organic compounds must contain the element