DCI 2 1 Chemical Equations Reactants and Products






- Slides: 17

DCI 2. 1 - Chemical Equations - Reactants and Products

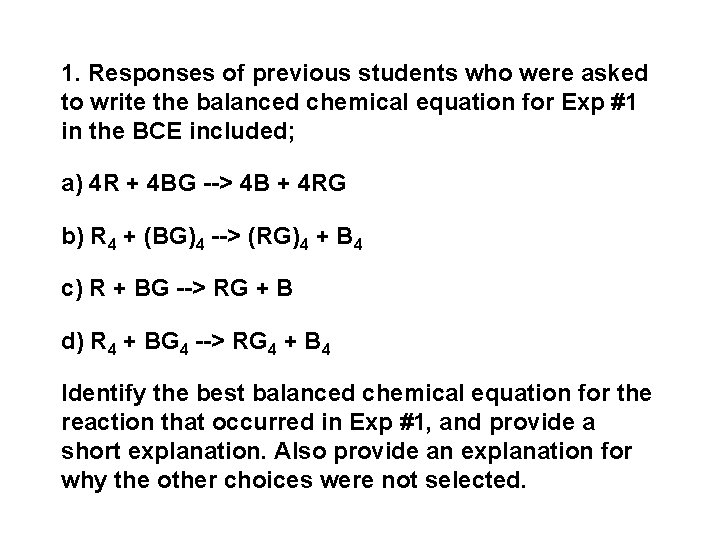
1. Responses of previous students who were asked to write the balanced chemical equation for Exp #1 in the BCE included; a) 4 R + 4 BG --> 4 B + 4 RG b) R 4 + (BG)4 --> (RG)4 + B 4 c) R + BG --> RG + B d) R 4 + BG 4 --> RG 4 + B 4 Identify the best balanced chemical equation for the reaction that occurred in Exp #1, and provide a short explanation. Also provide an explanation for why the other choices were not selected.

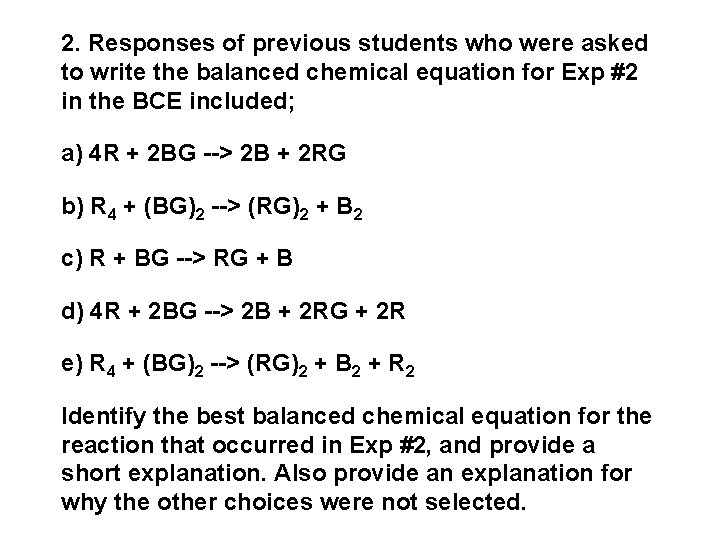
2. Responses of previous students who were asked to write the balanced chemical equation for Exp #2 in the BCE included; a) 4 R + 2 BG --> 2 B + 2 RG b) R 4 + (BG)2 --> (RG)2 + B 2 c) R + BG --> RG + B d) 4 R + 2 BG --> 2 B + 2 RG + 2 R e) R 4 + (BG)2 --> (RG)2 + B 2 + R 2 Identify the best balanced chemical equation for the reaction that occurred in Exp #2, and provide a short explanation. Also provide an explanation for why the other choices were not selected.

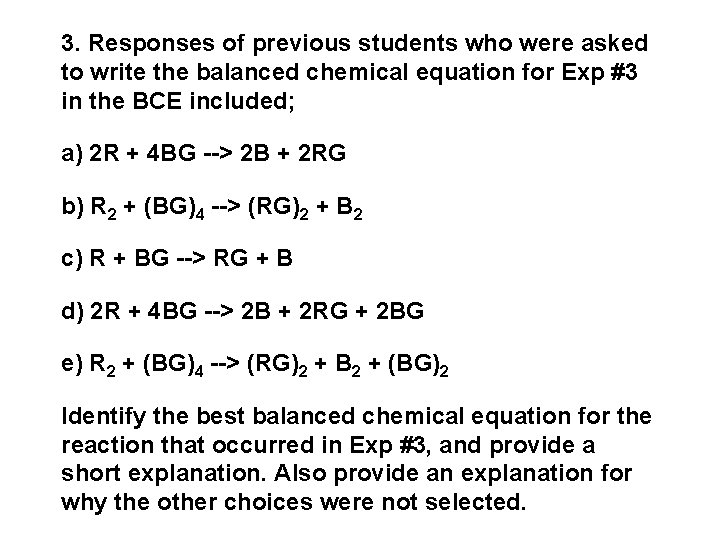
3. Responses of previous students who were asked to write the balanced chemical equation for Exp #3 in the BCE included; a) 2 R + 4 BG --> 2 B + 2 RG b) R 2 + (BG)4 --> (RG)2 + B 2 c) R + BG --> RG + B d) 2 R + 4 BG --> 2 B + 2 RG + 2 BG e) R 2 + (BG)4 --> (RG)2 + B 2 + (BG)2 Identify the best balanced chemical equation for the reaction that occurred in Exp #3, and provide a short explanation. Also provide an explanation for why the other choices were not selected.

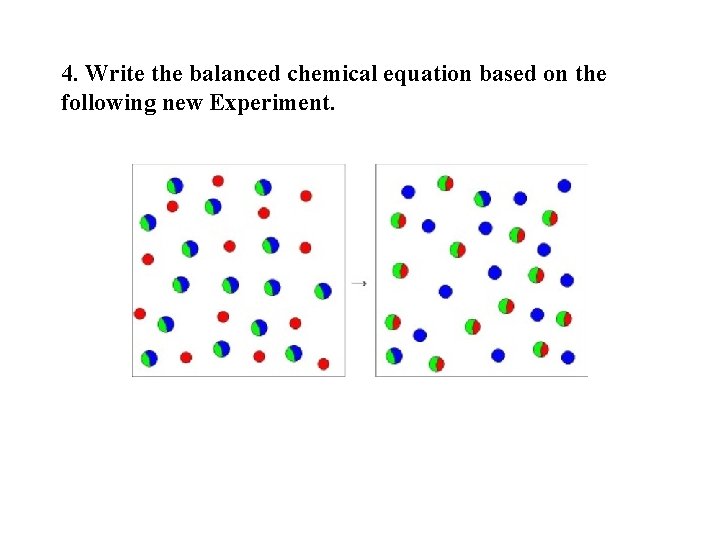
4. Write the balanced chemical equation based on the following new Experiment.

5. A reagent in excess is a reactant that is not completely used up in a chemical reaction, a limiting reagent is a reactant that limits the amount of product formed in a chemical reaction. Look back at Table II for Exp#1, #2 and #3 and select from below the statement that best fits the assignment of the excess reagent and the limiting reagent.

In Exp #1: a) R is the excess reagent and BG is the limiting reagent: b) BG is the excess reagent and R is the limiting reagent: c) both R and BG are excess reagents: d) both R and BG are limiting reagents

In Exp #2: a) R is the excess reagent and BG is the limiting reagent: b) BG is the excess reagent and R is the limiting reagent: c) both R and BG are excess reagents: d) both R and BG are limiting reagent

In Exp #3: a) R is the excess reagent and BG is the limiting reagent: b) BG is the excess reagent and R is the limiting reagent: c) both R and BG are excess reagents: d) both R and BG are limiting reagents

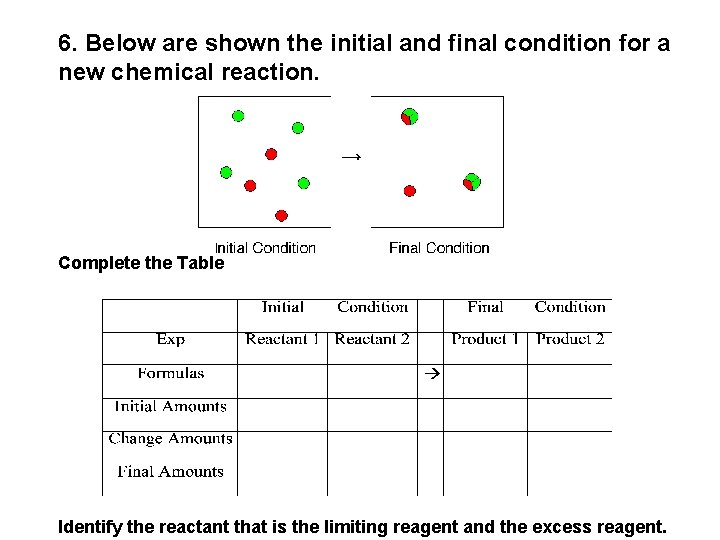
6. Below are shown the initial and final condition for a new chemical reaction. Complete the Table Identify the reactant that is the limiting reagent and the excess reagent.

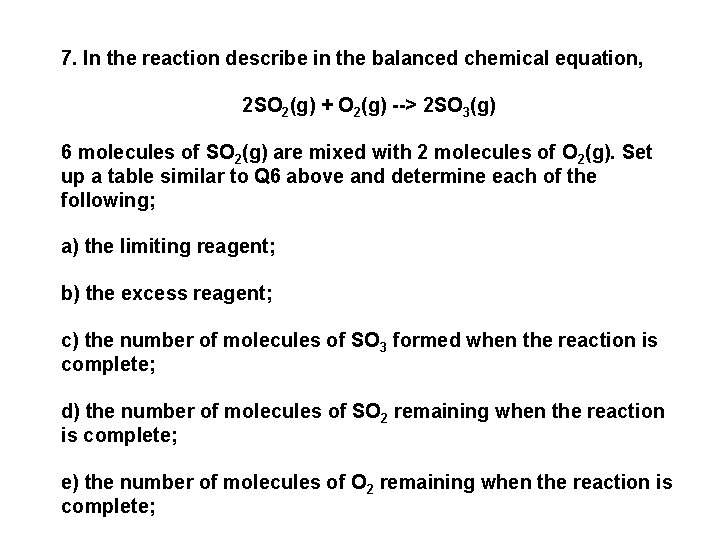
7. In the reaction describe in the balanced chemical equation, 2 SO 2(g) + O 2(g) --> 2 SO 3(g) 6 molecules of SO 2(g) are mixed with 2 molecules of O 2(g). Set up a table similar to Q 6 above and determine each of the following; a) the limiting reagent; b) the excess reagent; c) the number of molecules of SO 3 formed when the reaction is complete; d) the number of molecules of SO 2 remaining when the reaction is complete; e) the number of molecules of O 2 remaining when the reaction is complete;

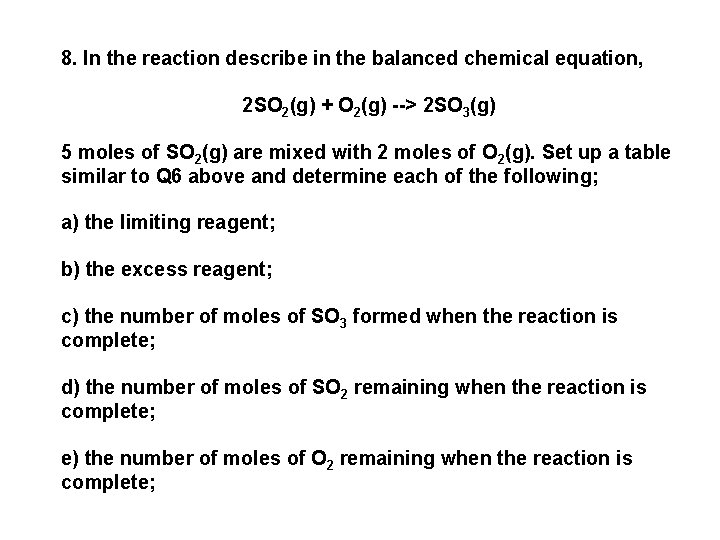
8. In the reaction describe in the balanced chemical equation, 2 SO 2(g) + O 2(g) --> 2 SO 3(g) 5 moles of SO 2(g) are mixed with 2 moles of O 2(g). Set up a table similar to Q 6 above and determine each of the following; a) the limiting reagent; b) the excess reagent; c) the number of moles of SO 3 formed when the reaction is complete; d) the number of moles of SO 2 remaining when the reaction is complete; e) the number of moles of O 2 remaining when the reaction is complete;

Clicker Questions

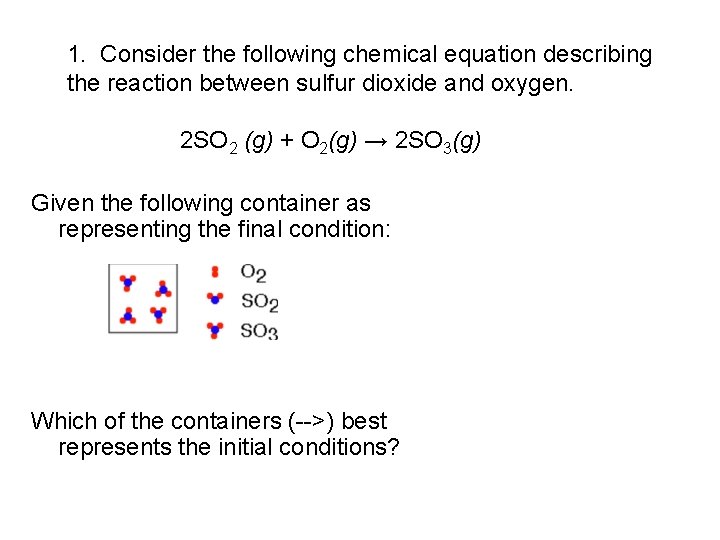
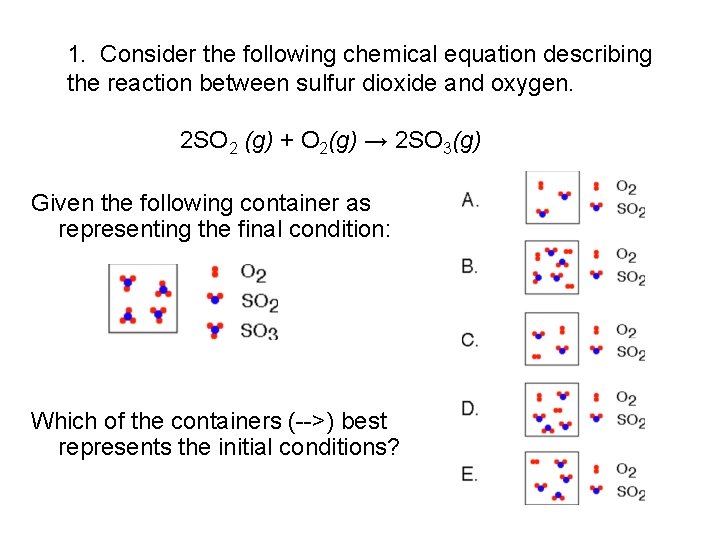
1. Consider the following chemical equation describing the reaction between sulfur dioxide and oxygen. 2 SO 2 (g) + O 2(g) → 2 SO 3(g) Given the following container as representing the final condition: Which of the containers (-->) best represents the initial conditions?

1. Consider the following chemical equation describing the reaction between sulfur dioxide and oxygen. 2 SO 2 (g) + O 2(g) → 2 SO 3(g) Given the following container as representing the final condition: Which of the containers (-->) best represents the initial conditions?

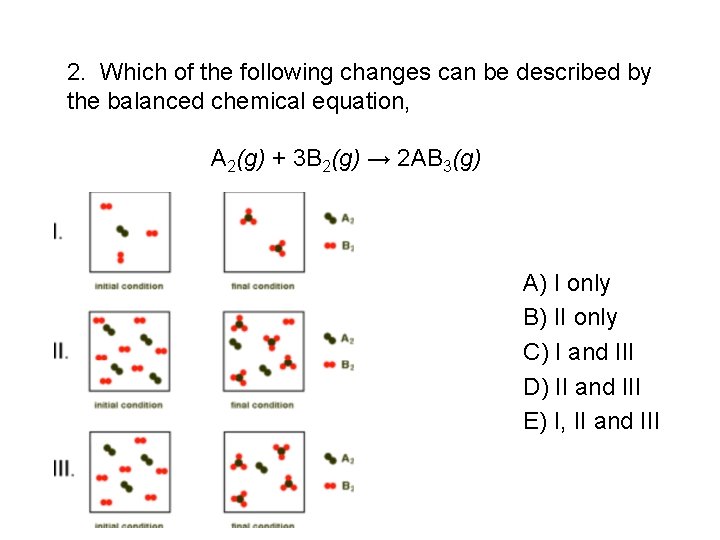
2. Which of the following changes can be described by the balanced chemical equation, A 2(g) + 3 B 2(g) → 2 AB 3(g) A) I only B) II only C) I and III D) II and III E) I, II and III

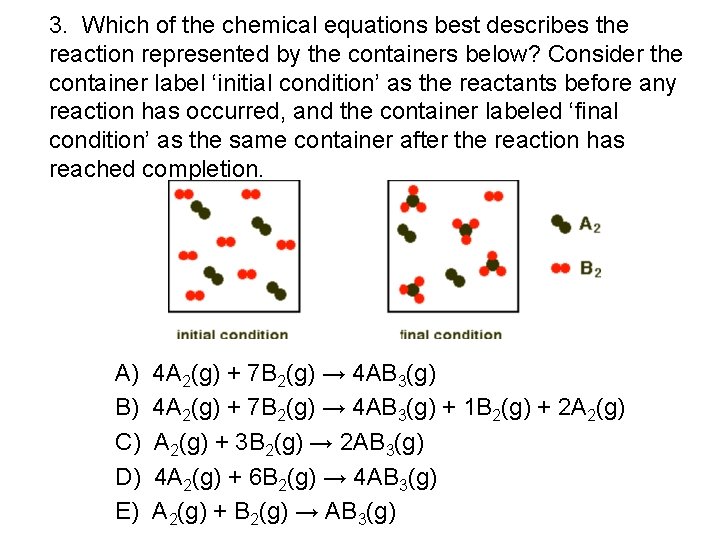
3. Which of the chemical equations best describes the reaction represented by the containers below? Consider the container label ‘initial condition’ as the reactants before any reaction has occurred, and the container labeled ‘final condition’ as the same container after the reaction has reached completion. A) B) C) D) E) 4 A 2(g) + 7 B 2(g) → 4 AB 3(g) + 1 B 2(g) + 2 A 2(g) + 3 B 2(g) → 2 AB 3(g) 4 A 2(g) + 6 B 2(g) → 4 AB 3(g) A 2(g) + B 2(g) → AB 3(g)