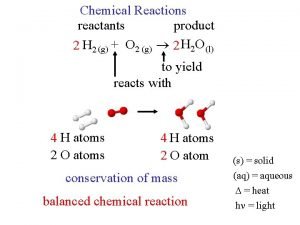
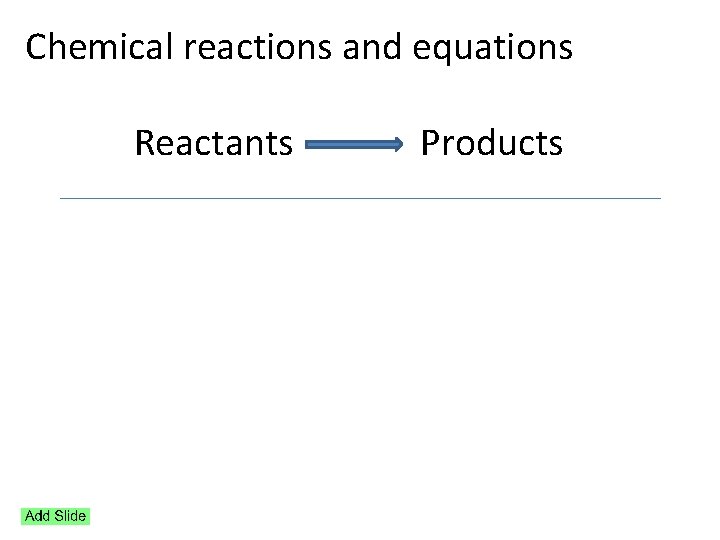
Reaction Stoichiometry Chemical reactions and equations Reactants Products




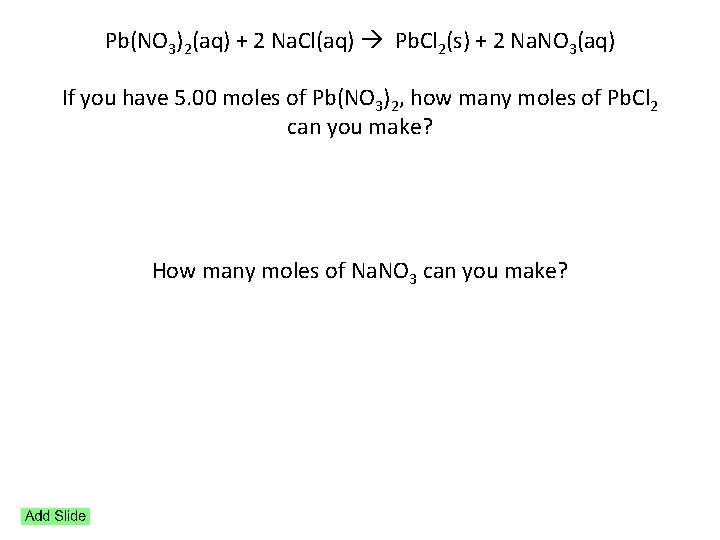


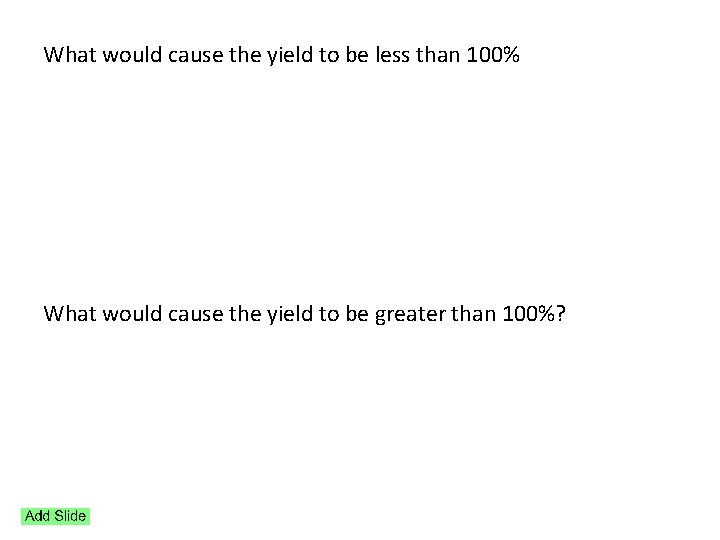





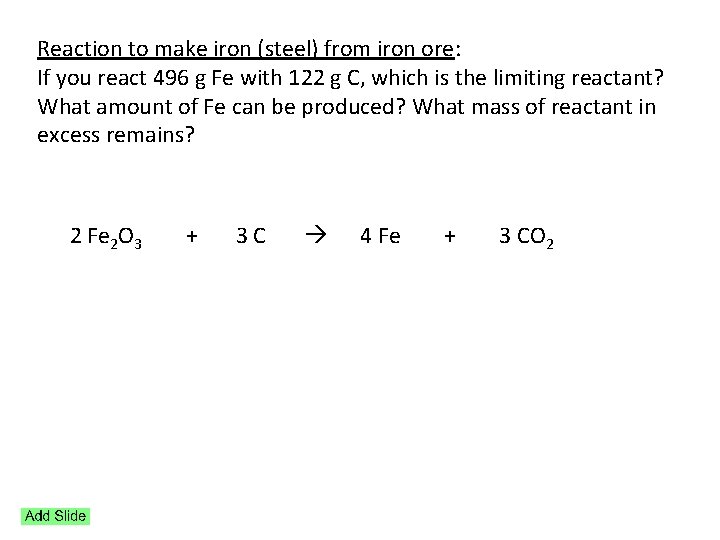










- Slides: 38

Reaction Stoichiometry

Chemical reactions and equations Reactants Products

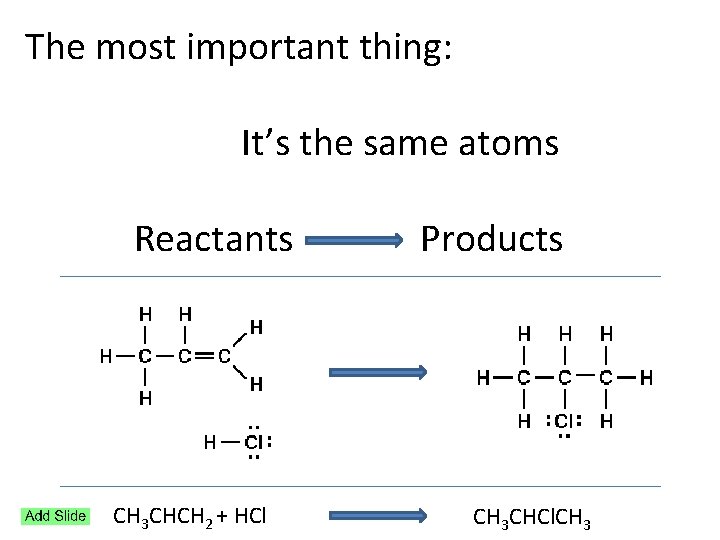
Chemical reactions and equations Reactants Products CH 3 CHCH 2 + HCl CH 3 CHCl. CH 3

The most important thing: It’s the same atoms Reactants Products CH 3 CHCH 2 + HCl CH 3 CHCl. CH 3

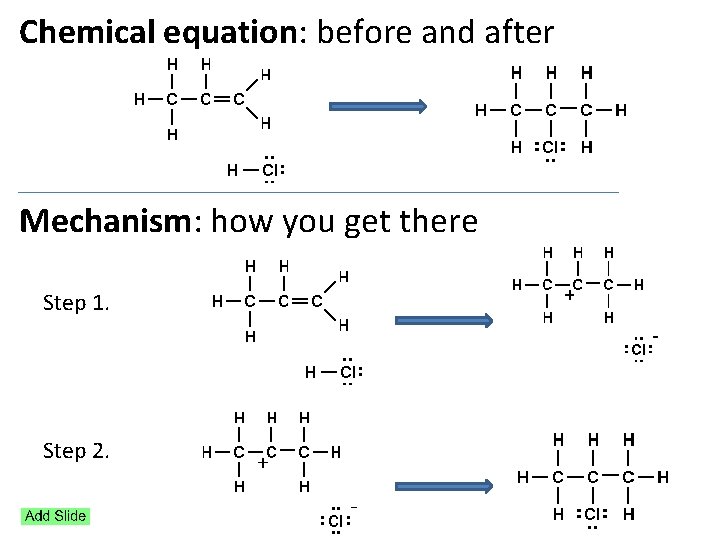
Chemical equation: before and after Mechanism: how you get there Step 1. Step 2.

Balancing Equations: Use models CH 3 OH + O 2

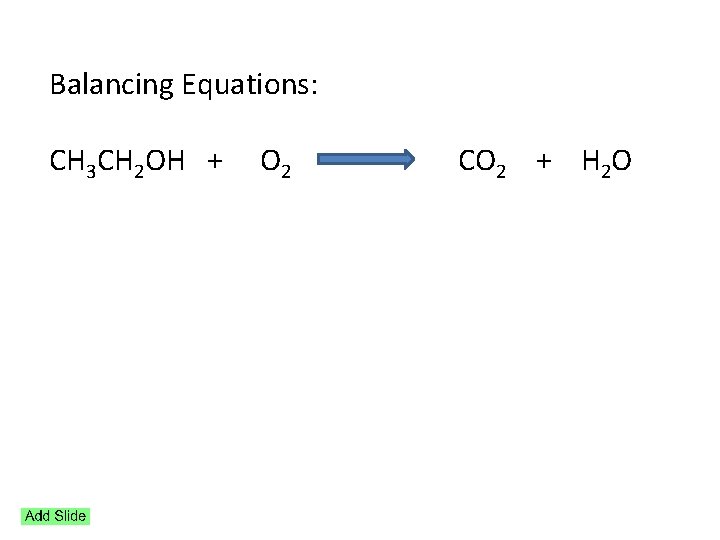
Balancing Equations: CH 3 CH 2 OH + O 2 CO 2 + H 2 O

Balancing Equations: North Korean Rocket Fuel (CH 3)2 NNH 2(l) + N 2 O 4(g) N 2(g) + H 2 O(g) + CO 2(g)

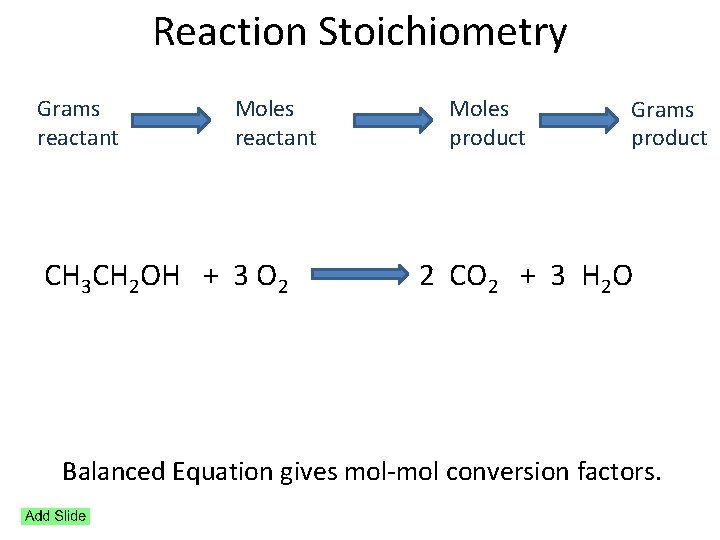
Reaction Stoichiometry Grams reactant Moles product Grams product CH 3 CH 2 OH + 3 O 2 2 CO 2 + 3 H 2 O Balanced Equation gives mol conversion factors.
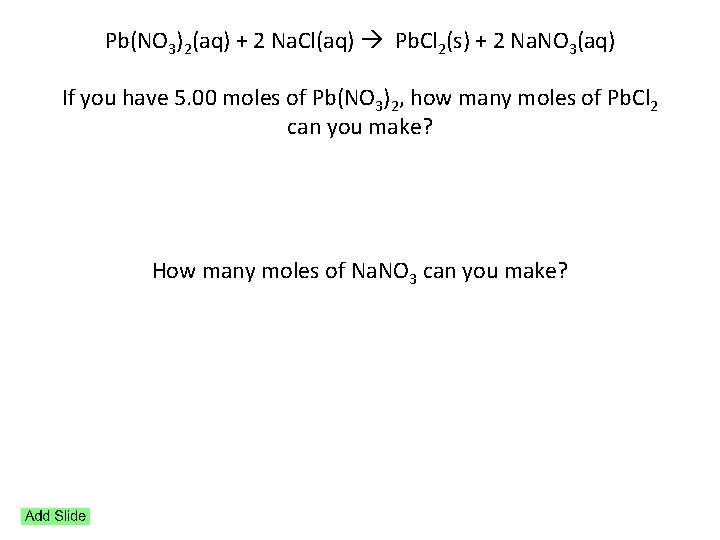
Pb(NO 3)2(aq) + 2 Na. Cl(aq) Pb. Cl 2(s) + 2 Na. NO 3(aq) If you have 5. 00 moles of Pb(NO 3)2, how many moles of Pb. Cl 2 can you make? How many moles of Na. NO 3 can you make?

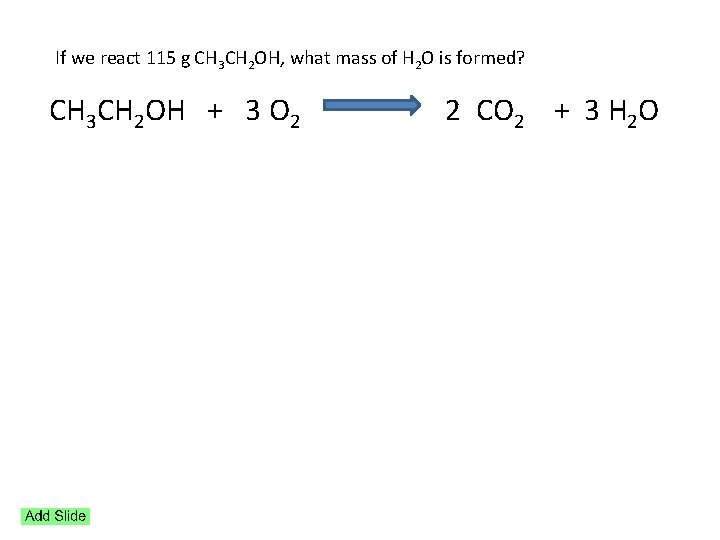
If we react 115 g CH 3 CH 2 OH, what mass of H 2 O is formed? CH 3 CH 2 OH + 3 O 2 2 CO 2 + 3 H 2 O

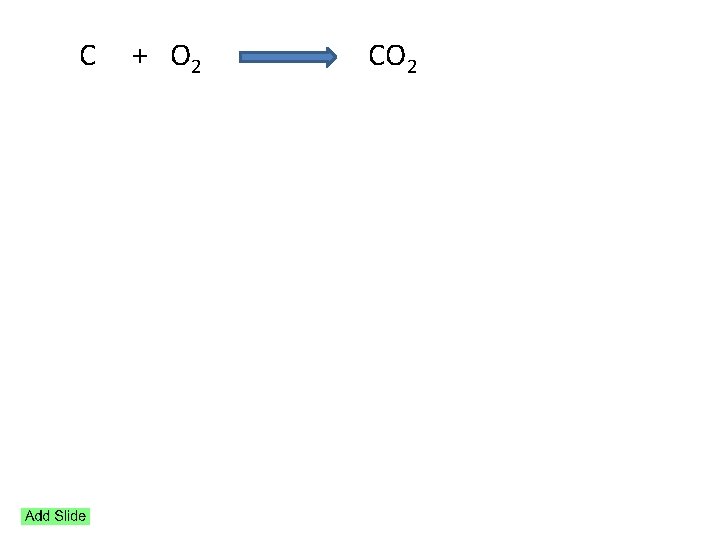
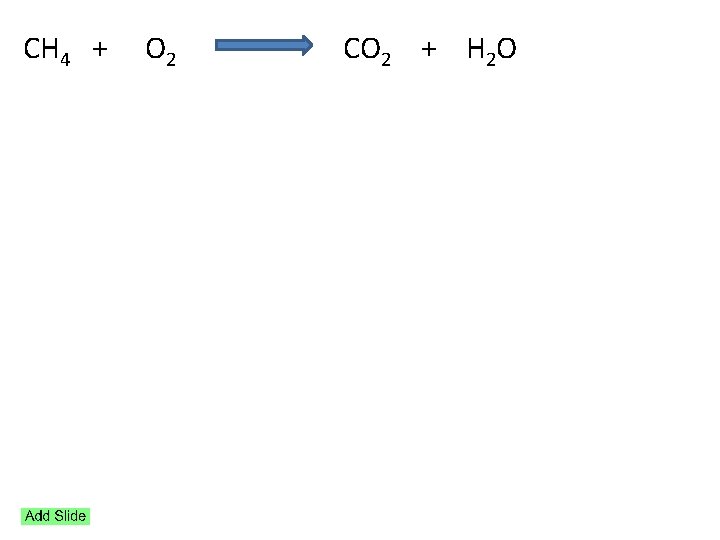
Fuel Comparison: Determine the mass of CO 2 formed from burning 1. 00 kg C, CH 4 and CH 3 CH 2 OH C + O 2 CO 2 CH 4 + O 2 CO 2 + H 2 O CH 3 CH 2 OH + O 2 CO 2 + H 2 O

C + O 2 CO 2

CH 4 + O 2 CO 2 + H 2 O

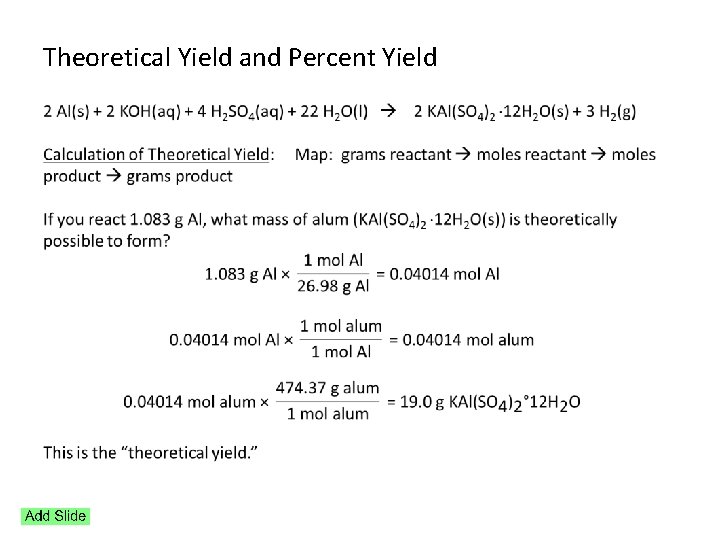
Theoretical Yield and Percent Yield

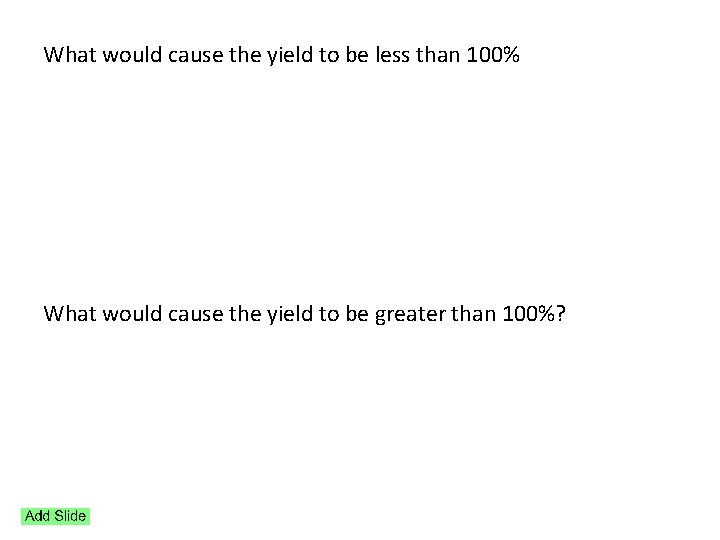
What would cause the yield to be less than 100% What would cause the yield to be greater than 100%?

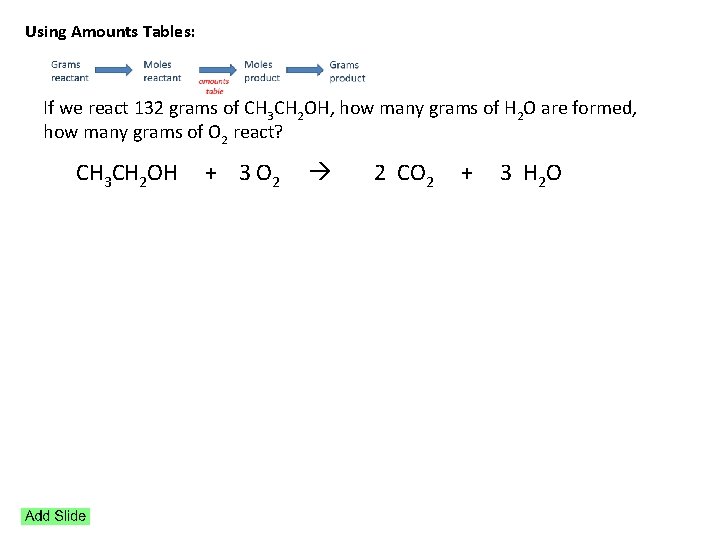
Using Amounts Tables: If we react 132 grams of CH 3 CH 2 OH, how many grams of H 2 O are formed, how many grams of O 2 react? CH 3 CH 2 OH + 3 O 2 2 CO 2 + 3 H 2 O


Limiting Reactants 1. If you have 200 tires and 75 steering wheels, how many cars can you make? 1. 200 2. 75 3. 50 4. 100

A cake recipe calls for 3 eggs, 2 cups of flour, and 3 teaspoons of sugar. If you have: • 9 eggs • 8 cups of flour • 12 teaspoons of sugar How many cakes can you make?


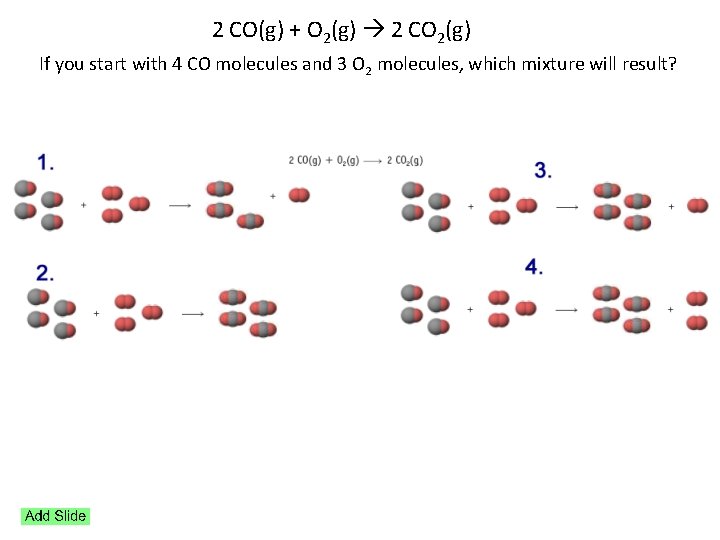
2 CO(g) + O 2(g) 2 CO 2(g) If you start with 4 CO molecules and 3 O 2 molecules, which mixture will result?

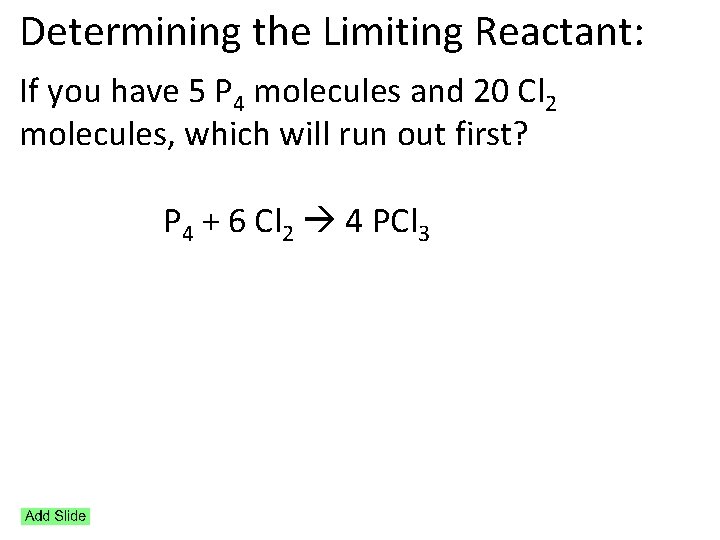
Determining the Limiting Reactant: If you have 5 P 4 molecules and 20 Cl 2 molecules, which will run out first? P 4 + 6 Cl 2 4 PCl 3

Limiting Reactants Simulation Course Website Simulations Chapter 3/Limiting Reactants
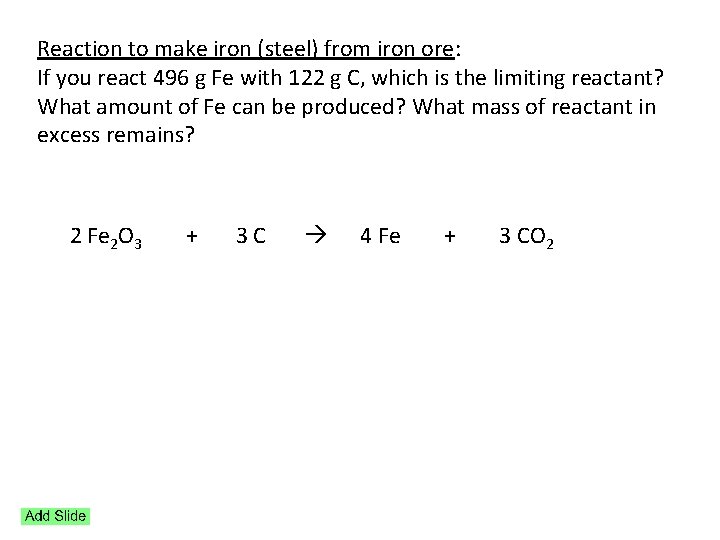
Reaction to make iron (steel) from iron ore: If you react 496 g Fe with 122 g C, which is the limiting reactant? What amount of Fe can be produced? What mass of reactant in excess remains? 2 Fe 2 O 3 + 3 C 4 Fe + 3 CO 2

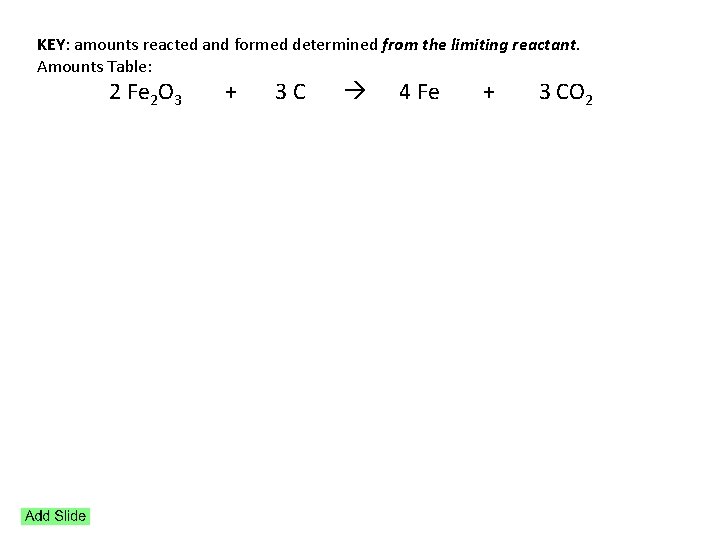
KEY: amounts reacted and formed determined from the limiting reactant. Amounts Table: 2 Fe 2 O 3 + 3 C 4 Fe + 3 CO 2

KEY: amounts reacted and formed determined from the limiting reactant. Amounts Table: 2 Fe 2 O 3 + 3 C 4 Fe + 3 CO 2

Amounts Tables 2 CO(g) + O 2(g) 2 CO 2(g) Initial Change Final

A hard somewhat Mind. Tap Question 14. 3 g CO react with 6. 48 g H 2 O to form H 2 and CO 2. What are the masses of all species following the reaction?

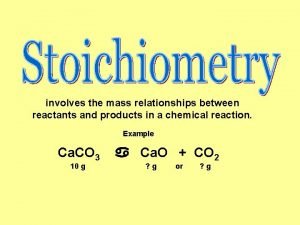
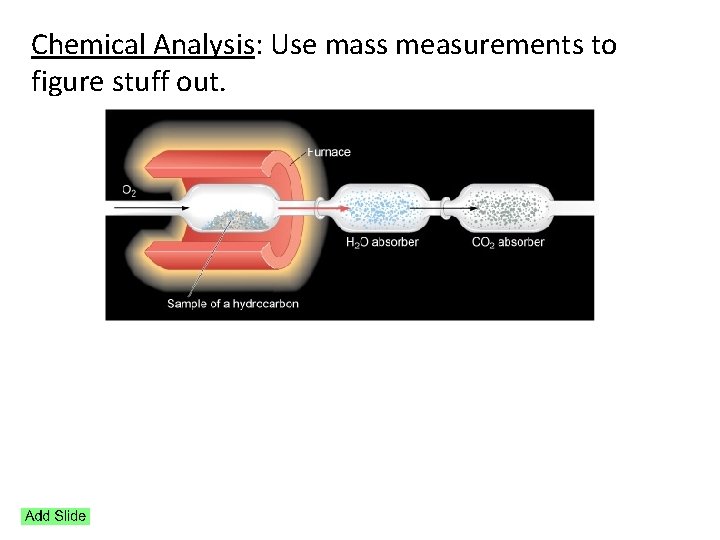
Chemical Analysis: Use mass measurements to figure stuff out.

A 5. 486 gram sample of a compound containing C, H and O is analyzed by combustion analysis and 9. 136 grams of CO 2 and 2. 993 grams of H 2 O are produced. What is the empirical formula?

A 5. 486 gram sample of a compound containing C, H and O is analyzed by combustion analysis and 9. 136 grams of CO 2 and 2. 993 grams of H 2 O are produced. What is the empirical formula?

A 5. 486 gram sample of a compound containing C, H and O is analyzed by combustion analysis and 9. 136 grams of CO 2 and 2. 993 grams of H 2 O are produced. What is the empirical formula?

Chemical Analysis A 8. 878 gram sample of manganese is heated in the presence of excess sulfur. A metal sulfide is formed with a mass of 14. 06 g. Determine the empirical formula of the metal sulfide.


A sample of a substance with the empirical formula XCl 2 weighs 0. 5539 g. When it is dissolved in water and reacted with Ag. NO 3, all its chlorine is converted to insoluble Ag. Cl. The mass of the resulting Ag. Cl is found to be 1. 1807 g. The chemical reaction is XCl 2 + 2 Ag. NO 3 2 Ag. Cl + X(NO 3)2 (a) Calculate the formula mass of XCl 2. molar mass XCl 2 = _____ g mol 1 (b) Calculate the molar mass of X. Atomic mass X = g mol 1 X is an unknown element

 Chemical reactions reactants and products
Chemical reactions reactants and products Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry What are the reactants in this chemical reaction?
What are the reactants in this chemical reaction? Stoichiometry map for chemical reactions
Stoichiometry map for chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions In a chemical reaction stoichiometry refers to
In a chemical reaction stoichiometry refers to Predicting products of chemical reactions
Predicting products of chemical reactions Predicting products of chemical reactions
Predicting products of chemical reactions More predicting products of chemical reactions
More predicting products of chemical reactions Section 3 predicting the products of chemical reactions
Section 3 predicting the products of chemical reactions Chemistry chapter 8 review chemical equations and reactions
Chemistry chapter 8 review chemical equations and reactions Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Chemical equations and reactions chapter 8 review
Chemical equations and reactions chapter 8 review Hcl and sodium hydrogen carbonate
Hcl and sodium hydrogen carbonate Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Quantitative study of reactants and products
Quantitative study of reactants and products Phet products reactants and leftovers
Phet products reactants and leftovers Enthalpy of products
Enthalpy of products Chemical equation symbols
Chemical equation symbols What are the reactants and products of photosynthesis?
What are the reactants and products of photosynthesis? Reactants, products and leftovers
Reactants, products and leftovers Elephant toothpaste reactants and products
Elephant toothpaste reactants and products Reactants products and leftovers
Reactants products and leftovers Reactants and products
Reactants and products Relationship between reactants and products
Relationship between reactants and products Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Lesson 68 toxic reactions chemical equations answer key
Lesson 68 toxic reactions chemical equations answer key Chapter 19 chemical reactions answer key
Chapter 19 chemical reactions answer key Toxic reactions chemical equations
Toxic reactions chemical equations Neutron emission
Neutron emission Aqueous reactions and solution stoichiometry
Aqueous reactions and solution stoichiometry Why are average bond enthalpies inexact values
Why are average bond enthalpies inexact values Enthalpy diagram worksheet
Enthalpy diagram worksheet When does a chemical reaction stop
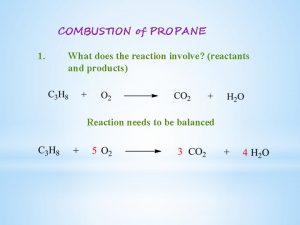
When does a chemical reaction stop Combustion reaction reactants
Combustion reaction reactants