Cornell Notes 2110 What weve learned so far
















- Slides: 37

Cornell Notes 2/1/10

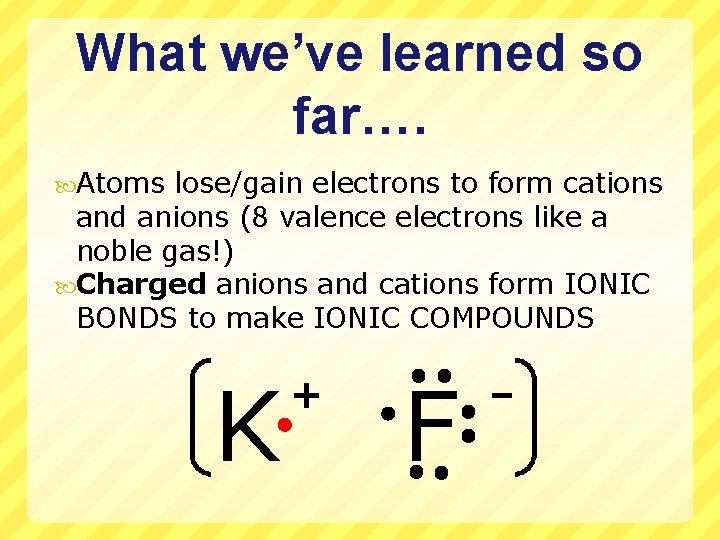
What we’ve learned so far…. Atoms lose/gain electrons to form cations and anions (8 valence electrons like a noble gas!) Charged anions and cations form IONIC BONDS to make IONIC COMPOUNDS K + F -

What we’re learning today… Atoms can also SHARE electrons to form COVALENT BONDS! Why is this important? Covalent bonds are important in many organic molecules (living creatures) Examples: Cell membranes are made of lipids (We’d be puddles of liquid without them!) Proteins (We’d starve without them!)

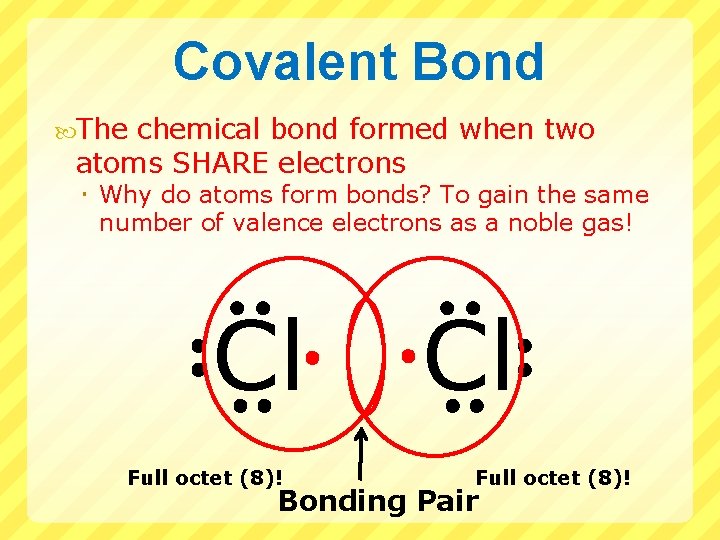
Covalent Bond The chemical bond formed when two atoms SHARE electrons Why do atoms form bonds? To gain the same number of valence electrons as a noble gas! Cl Full octet (8)! Bonding Pair

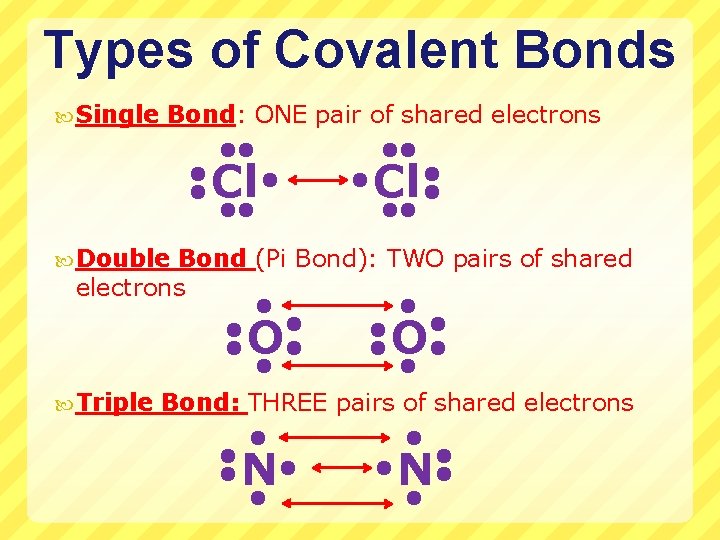
Types of Covalent Bonds Single Bond: ONE pair of shared electrons Cl Cl Double Bond (Pi Bond): TWO pairs of shared electrons O Triple O Bond: THREE pairs of shared electrons N N

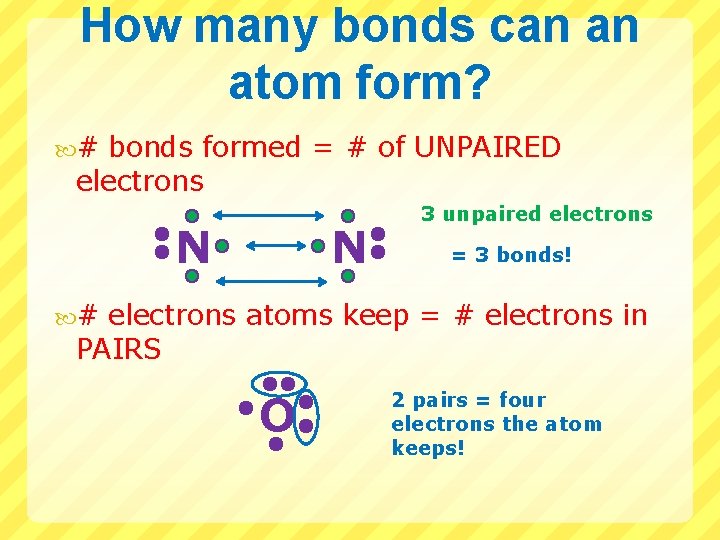
How many bonds can an atom form? # bonds formed = # of UNPAIRED electrons N N 3 unpaired electrons = 3 bonds! # electrons atoms keep = # electrons in PAIRS O 2 pairs = four electrons the atom keeps!

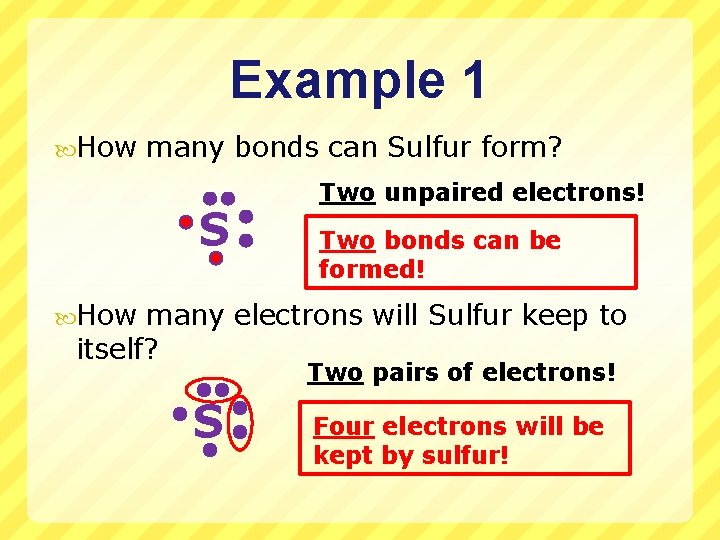
Example 1 How many bonds can Sulfur form? S Two unpaired electrons! Two bonds can be formed! How many electrons will Sulfur keep to itself? Two pairs of electrons! S Four electrons will be kept by sulfur!

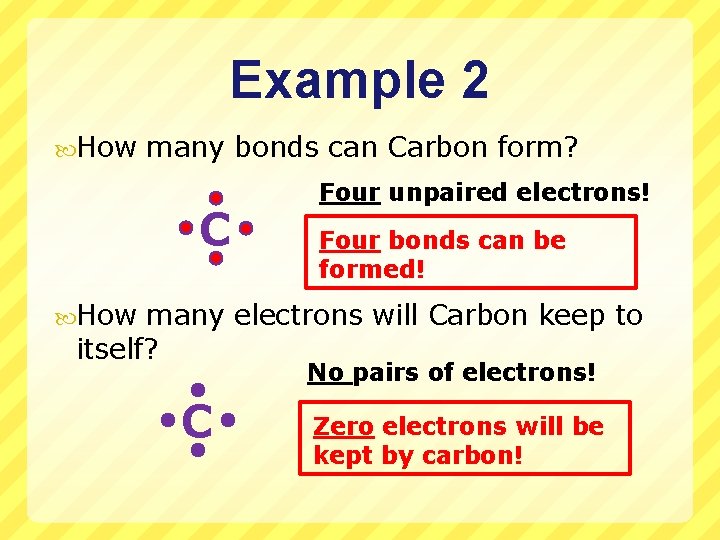
Example 2 How many bonds can Carbon form? C Four unpaired electrons! Four bonds can be formed! How many electrons will Carbon keep to itself? No pairs of electrons! C Zero electrons will be kept by carbon!

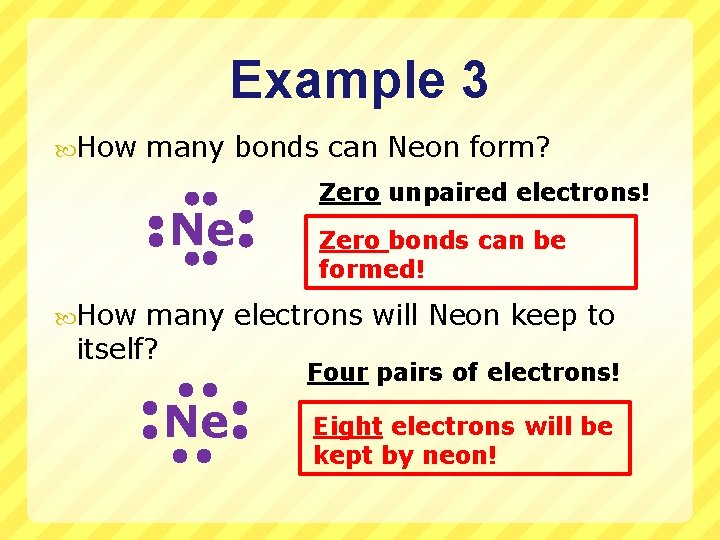
Example 3 How many bonds can Neon form? Ne Zero unpaired electrons! Zero bonds can be formed! How many electrons will Neon keep to itself? Four pairs of electrons! Ne Eight electrons will be kept by neon!

Cornell Notes

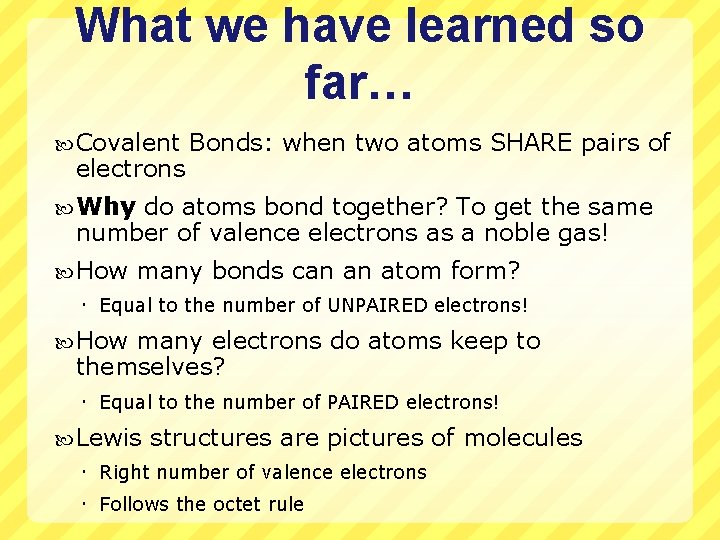
What we have learned so far… Covalent electrons Bonds: when two atoms SHARE pairs of Why do atoms bond together? To get the same number of valence electrons as a noble gas! How many bonds can an atom form? Equal to the number of UNPAIRED electrons! How many electrons do atoms keep to themselves? Equal to the number of PAIRED electrons! Lewis structures are pictures of molecules Right number of valence electrons Follows the octet rule

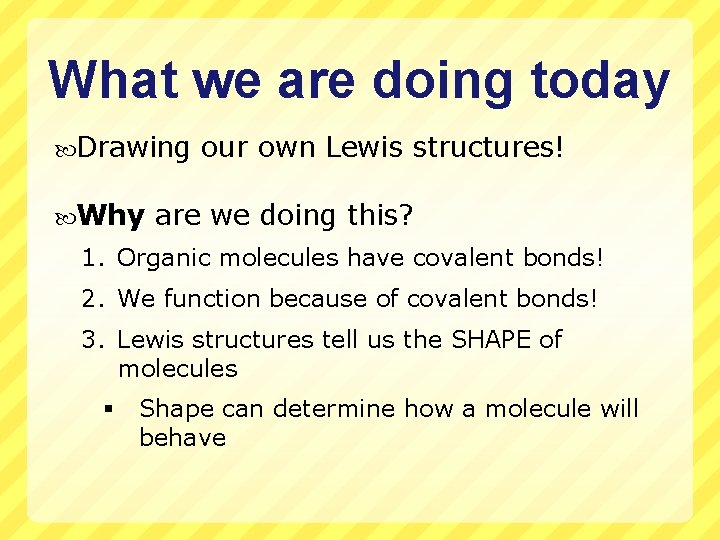
What we are doing today Drawing Why our own Lewis structures! are we doing this? 1. Organic molecules have covalent bonds! 2. We function because of covalent bonds! 3. Lewis structures tell us the SHAPE of molecules § Shape can determine how a molecule will behave

How do we draw a Lewis structure? 1. Determine what elements and how many atoms you have in a molecule from the formula 2. Draw the electron dot structures for every atom The atom(s) with the most unpaired electrons go in the middle 3. Connect the dots! (unpaired electrons) 4. Redraw the structure neatly so lines are straight

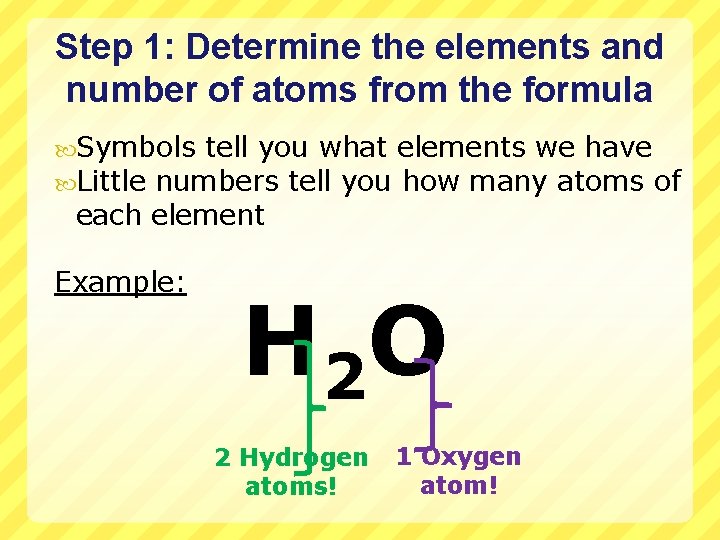
Step 1: Determine the elements and number of atoms from the formula Symbols tell you what elements we have Little numbers tell you how many atoms of each element Example: H 2 O 2 Hydrogen atoms! 1 Oxygen atom!

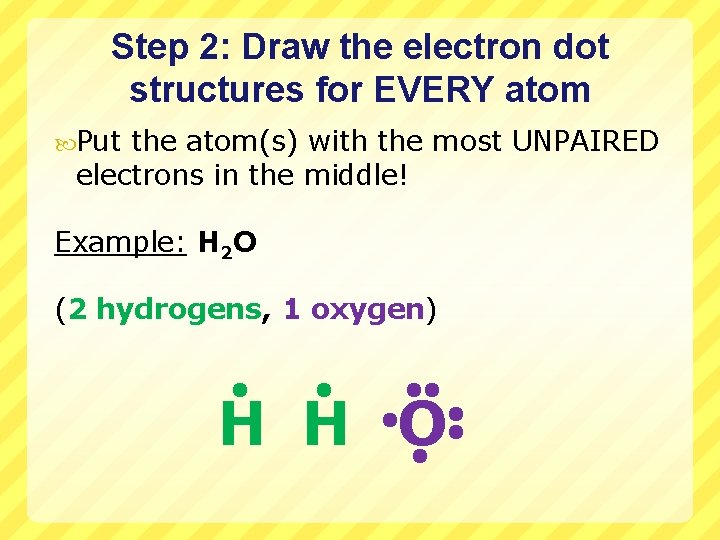
Step 2: Draw the electron dot structures for EVERY atom Put the atom(s) with the most UNPAIRED electrons in the middle! Example: H 2 O (2 hydrogens, 1 oxygen) H H O

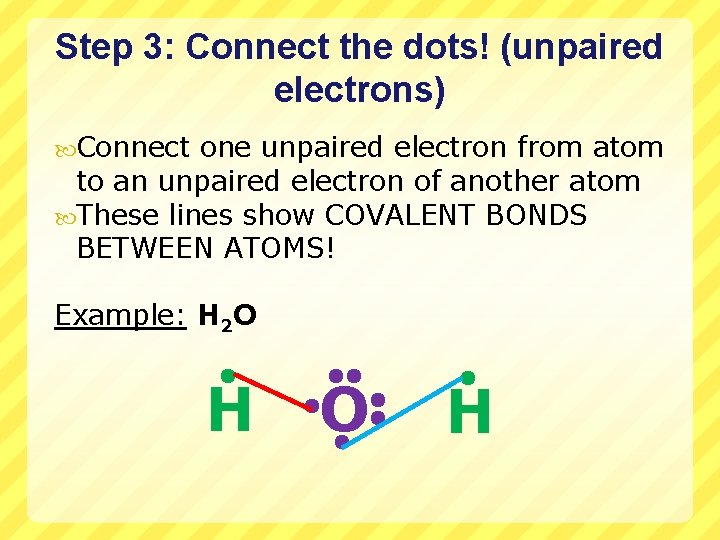
Step 3: Connect the dots! (unpaired electrons) Connect one unpaired electron from atom to an unpaired electron of another atom These lines show COVALENT BONDS BETWEEN ATOMS! Example: H 2 O H

Step 4: Redraw the structure Lines are straight Leave paired electrons in the structure Example: H 2 O H H O H

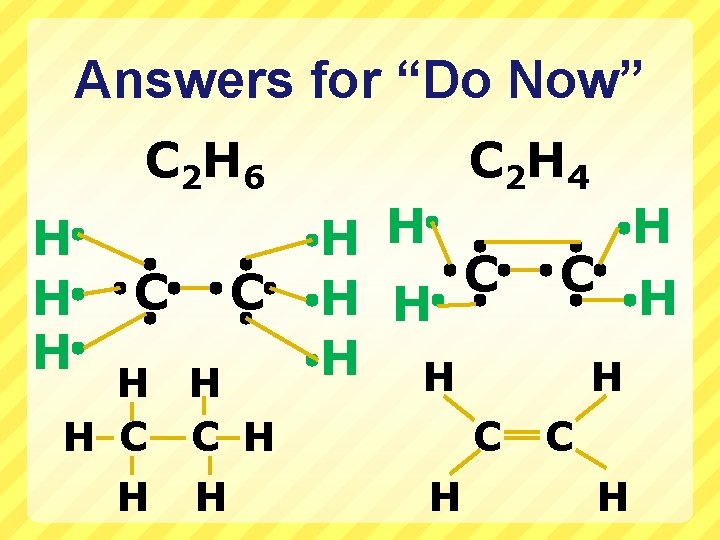
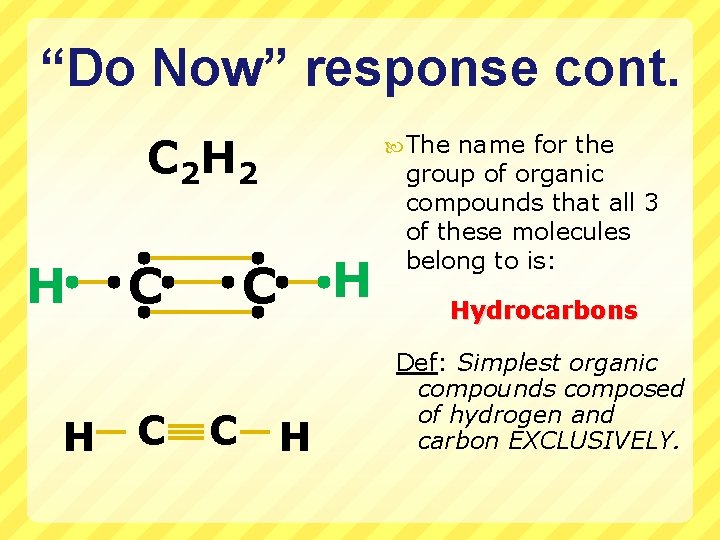
“Do Now” for 2/22/10 Draw the Lewis structures for the following molecules (look in your notes or p. 254 in your text) C 2 H 6 (common name ‘ethane’, an alkane) C 2 H 4 (common name ‘ethylene’, an alkene) C 2 H 2 (common name ‘acetylene’, an alkyne) Bonus Point: What is the special name for the group of compounds that these 3 molecules fall under? When you are done, QUICKLY finish drawing the compounds from Friday in your notes (or on your worksheet) so we can go over them: H 2 S H 2 O 2 NH 2 Cl HCN

Answers for “Do Now” C 2 H 6 H H H C C H H H C 2 H 4 H H C H H C H

“Do Now” response cont. C 2 H H C C The H C C H name for the group of organic compounds that all 3 of these molecules belong to is: Hydrocarbons Def: Simplest organic compounds composed of hydrogen and carbon EXCLUSIVELY.

Announcements We will be finishing Ch. 8 this week If by the end of class on Wed. you are struggling with ionic or covalent bonds, it is highly recommended that you attend an after school review session Response to lab ? ’s are due TODAY New HW was posted in class and online last Fri and is due on Thurs, 2/25 Exam on Ch. 7 and 8 is scheduled for THIS Friday, 2/26, review guides on table – complete by Thursday! You do NOT need to complete the graphic organizer for steps to naming covalent compounds (Ch. 8. 2 HW). You may for EC if you like. You NEED your book Tues-Thurs. No book = no chair for you! (until you have a chance to correctly answer a ? at least)

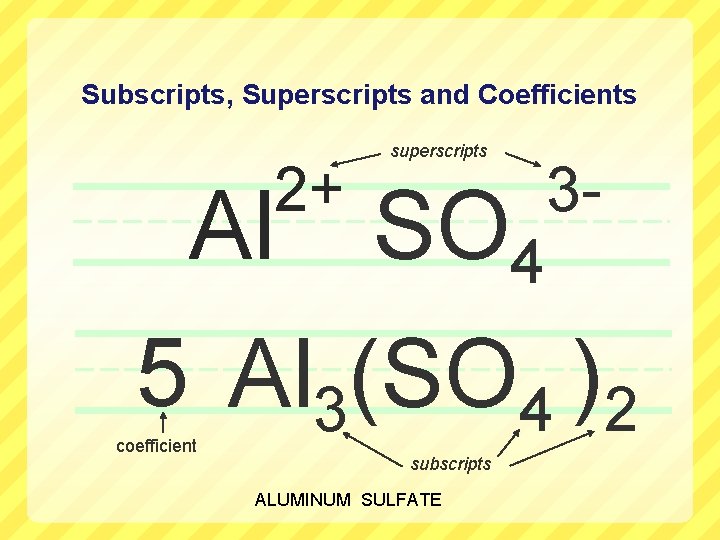
Subscripts, Superscripts and Coefficients Al 2+ superscripts SO 4 3 - 5 Al 3(SO 4 )2 coefficient subscripts ALUMINUM SULFATE

Title: Ch. 8 Overview Cornell Notes Review: What is the difference between a compound a molecule? Compound: term used to describe elements that have ionic bonds chemical combination of 2 or more different elements can be broken down into simpler substances has properties different than those of the elements which make it up Molecule: term used to describe atoms of either the same or different elements that share e- (are covalently bonded)

Strength of covalent bonds Bond Length Def: Distance from the center of 1 nucleus to the center of another between bonded atoms. increases bond As # of shared e- pairs _____, decreases length_____. decreases the strength of the As bond length _____, increases bond _____. Bonds and energy If you form a bond energy is released. If you break a bond energy must be added, or used. The energy required to break a bond is called bond-dissociation energy.

Molecular Structures Quickly sketch the graphic below of the different types of models for showing molecules in your notes.

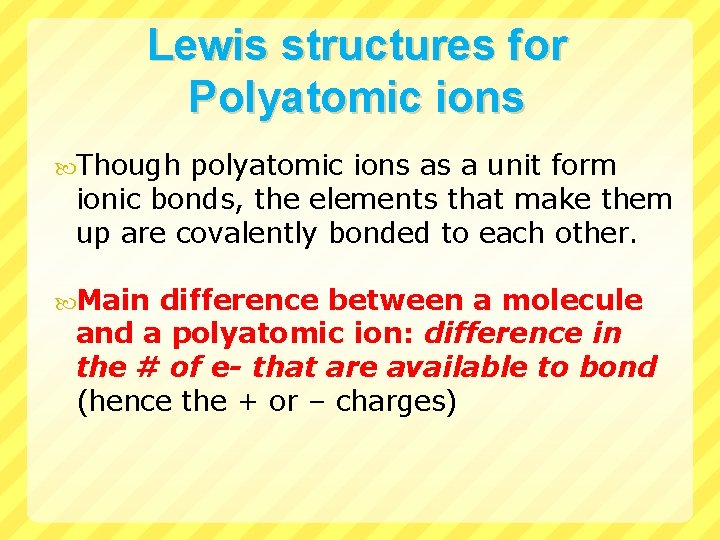
Lewis structures for Polyatomic ions Though polyatomic ions as a unit form ionic bonds, the elements that make them up are covalently bonded to each other. Main difference between a molecule and a polyatomic ion: difference in the # of e- that are available to bond (hence the + or – charges)

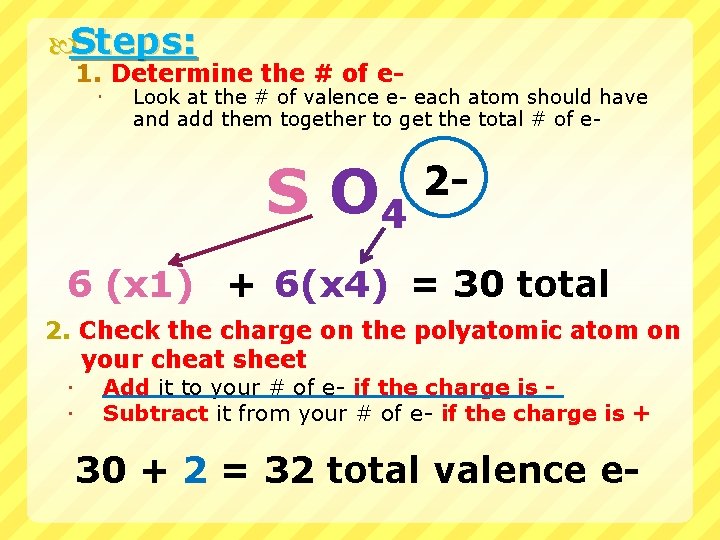
Steps: 1. Determine the # of e Look at the # of valence e- each atom should have and add them together to get the total # of e- S O 4 2 - 6 (x 1) + 6(x 4) = 30 total 2. Check the charge on the polyatomic atom on your cheat sheet Add it to your # of e- if the charge is Subtract it from your # of e- if the charge is + 30 + 2 = 32 total valence e-

3. Determine which will be the central atom (Remember – it will be the atom that wants to make the most bonds!) • Both S and O have ____ 6 valence e-, so both want to make ____ 2 bonds. • So what do we do? ? • The element with the lowest electronegativity will be the central atom. • • Remember: electronegativity is an atom’s ability to attract e. Look at p. 194 in Ch. 6. 3 for the trend…which you SHOULD have memorized! In this case, it is S • General rules: • H will always be a terminal atom • 1 st element listed will usually be the central atom

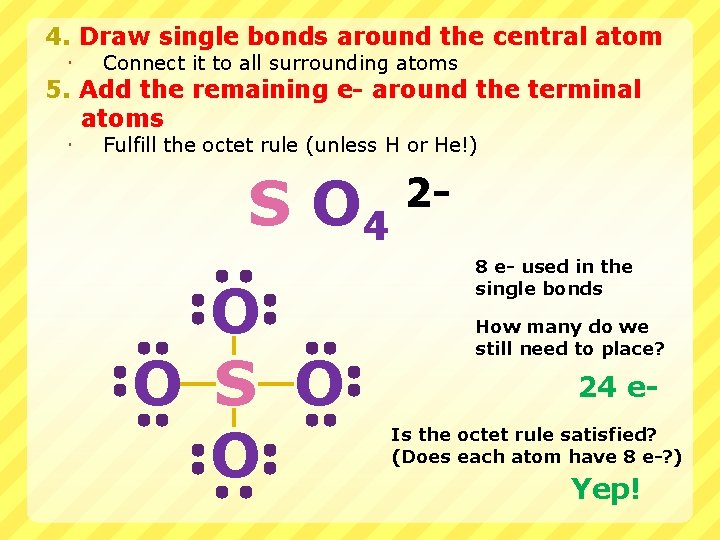
4. Draw single bonds around the central atom Connect it to all surrounding atoms Fulfill the octet rule (unless H or He!) 5. Add the remaining e- around the terminal atoms S O 4 O O S O O 28 e- used in the single bonds How many do we still need to place? 24 e. Is the octet rule satisfied? (Does each atom have 8 e-? ) Yep!

“Do Now” for 2/23 Which of the following molecules has the strongest bonds? 1. 1 st draw the Lewis structures for the molecules 2 nd look back over your notes to see how to determine bond strength. (p. 246 in text if you were absent) a. ) CN- C b. ) CH 3 NH 2 N As the # of shared pairs of eincreases, the bond length decreases, which means the bond is stronger. Hint: the C and the N are central atoms H H H C N H H

Naming Covalent Molecules Ch. 8. 2 Binary Molecular Compounds nonmetal atoms) (2 1. Name the first element in the compound 2. Name the 2 nd element using the root and the suffix “ide” (Just like in monatomic ionic compounds!) 3. Use prefixes to denote the # of each of the elements.

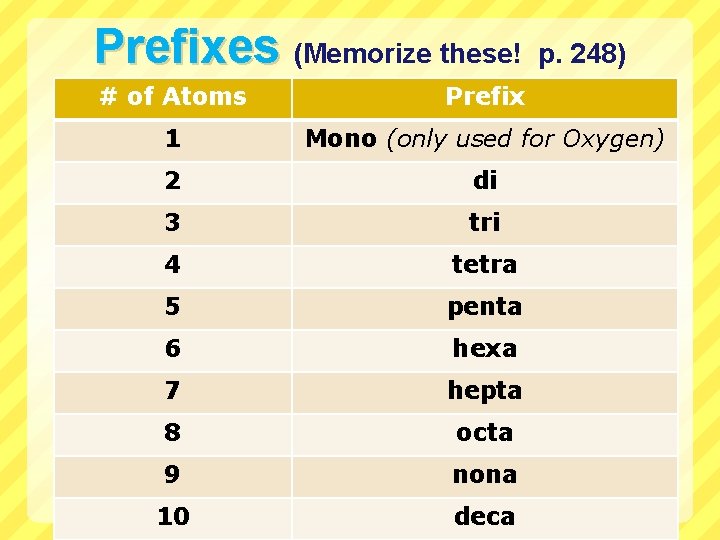
Prefixes (Memorize these! p. 248) # of Atoms Prefix 1 Mono (only used for Oxygen) 2 di 3 tri 4 tetra 5 penta 6 hexa 7 hepta 8 octa 9 nona 10 deca

Let’s Practice! (Answer in your notes) 1. CO 2 Carbon dioxide 2. SO 2 Sulfur dioxide 3. CCl 4 Carbon tetrachloride 3. P 2 O 5 diphosphorous pentoxide

Naming Binary Acids (H + 1 other element) Write these down!! HCl Add the prefix “hydro” to the name of the 2 nd element 2. Remainder of 1 st word is the root of the 2 nd element with the suffix “ic” 3. The 2 nd word is always acid. 1. Hydrochloric acid

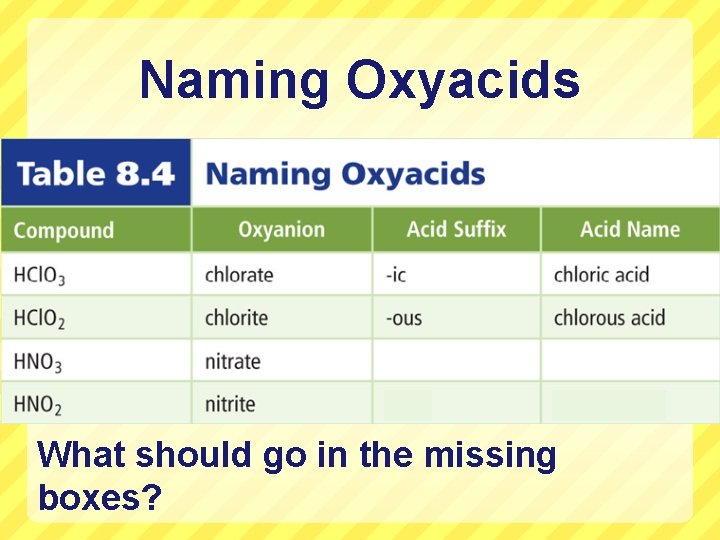
Naming Oxyacids (H + an oxyanion) Oxyanion = polyatomic more oxygen atoms. ion that contains 1 or HNO 3 Write these down!! 1. Identify the oxyanion present. 2. 1 st word of name begins w/ the root of 3. 4. the oxyanion (and the prefix “per” or “hypo” if it is part of the name) The suffix of the 1 st word is: “ic” if the oxyanion ends w/ “ate” “ous” if the oxyanion ends w/ “ite” The 2 nd word is always “acid”. Nitrate Nitr ic acid

Naming Oxyacids What should go in the missing boxes?

notes) 1. Say whether it is a binary or an oxyacid 2. Write the name of the acid following the proper steps 1. HI BINARY Hydroiodic acid 2. HCl. O 3 OXYACID Chloric acid 3. HCl. O 2 OXYACID Chlorous acid 4. H 2 SO 4 OXYACID Sulfuric acid 5. H 2 S BINARY Hydrosulfuric acid