Conversion factors from chemical formula Chemical formula contains

















- Slides: 18

Conversion factors from chemical formula Chemical formula contains information regarding the relationship between atoms(moles of atoms) or between molecules(moles of molecules) Example: The formula of CCl 2 F 2 tells us that there is 2 mole of Cl for every one mole of CCl 2 F 2

Practice Problem • Calculate the number of moles of Chlorine in 38. 5 mole of CCl 2 F 2 ?

Practice Problem • Calculate the mass in grams of Cl contained in 25 g of CCl 2 F 2 ?

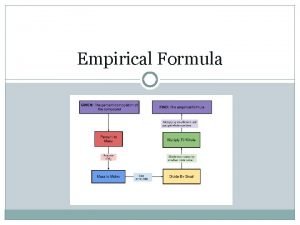
Obtaining an empirical formula from Experimental data • Steps 1. Write down the given masses of each element in the compound. If you are given mass percentage composition, assume a 100 g sample and calculate the masses of each element from the given percentage

Steps 2. Convert each mass in to moles by using appropriate molar mass for each element as a conversion factor 3. Write down the pseudo formula for the compound using the number of moles of each element(from step 2) as a subscript

Steps • 4. Divide all the subscripts in the formula by the smaller subscript • 5. If the subscripts are not whole numbers, multiply all the subscripts with a whole number to get whole number subscript

Practice • A compound containing N and O is decomposed in the laboratory and produces 24. 5 g N and 70. 0 g of Calculate the empirical formula of the compound

Practice • A laboratory analysis of Aspirin determined the following mass percent composition • C 60. 0% • H 4. 48% • O 35. 52% • Find the empirical formula

Practice Problem • A Sample of Compound is decomposed in the laboratory and produces 165 g of C, 27. 8 g of H and 220. 2 g of O calculate the empirical formula of the compound?

Practice Problem • Ibuprofen has the following mass percent composition • C – 75. 69% • H – 8. 80% • O – 15. 51% • What is the empirical formula of Ibuprofen?

Calculating molecular formula from Empirical formula • Molecular formula of a compound = Empirical formula × n • Where n = 1, 2, 3 ….

Calculating the molecular formula • n can be obtained by Molar mass ÷ Empirical formula molar mass • Calculate the Molecular formula for fructose? Given that its empirical formula is CH 2 O and its molar mass is 180. 2 g/mol

Problem • A compound has the empirical formula CH and a molar mass of 78. 11 g/ mol. What is its molecular formula?

Determine the molecular formula • A compound with a percentage composition shown next has a molar mass of 60. 1 g/mol. Determine its molecular formula? • C – 39. 97% • H – 13. 41% • N – 46. 62%

Combustion Analysis • Combustion analysis is another way to determine the empirical formula of an unknown compound. • Especially used to find the empirical formula of compounds containing C and H • When the sample is burned, all the C in the sample is converted to CO 2 and all the H is converted to H 2 O • The CO 2 and H 2 O produced are then weighed

Combustion Analysis • With the masses, we can use the numerical relationships between moles inherent in the formula for CO 2 and H 2 O to determine the masses of C and H in the original sample • 1 mol of CO 2 : 1 mol of C • 1 mol of H 2 O: 2 mol of H • The amount of Other elements can be determined by subtracting the sum of the masses of C and H from the original mass of the sample.


Topics Remaining 1. Obtaining an empirical formula from combustion analysis 2. How to write balanced chemical equations 3. Balancing chemical equation containing ionic compounds with polyatomic ions
 How to factor label
How to factor label Grid factor formula
Grid factor formula Common conversion factors
Common conversion factors Writing conversion factors
Writing conversion factors Equalities and conversion factors
Equalities and conversion factors 3 kilograms
3 kilograms 23 lbm.ft/min2 to kg.cm/s2
23 lbm.ft/min2 to kg.cm/s2 Conversion factors
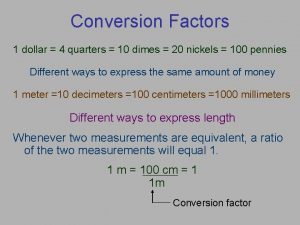
Conversion factors Conversion factors
Conversion factors 4 quarters is a dollar
4 quarters is a dollar Site vs situation factors
Site vs situation factors Biotic and abiotic drawing
Biotic and abiotic drawing Abiotic vs biotic factors
Abiotic vs biotic factors Aboitic environment
Aboitic environment Is sunlight biotic
Is sunlight biotic Site factors vs situation factors
Site factors vs situation factors What are the factors of 8
What are the factors of 8 Common factors of 10 and 20
Common factors of 10 and 20 8-1 factors and greatest common factors
8-1 factors and greatest common factors