Conversion Factors and Unit Cancellation A physical quantity


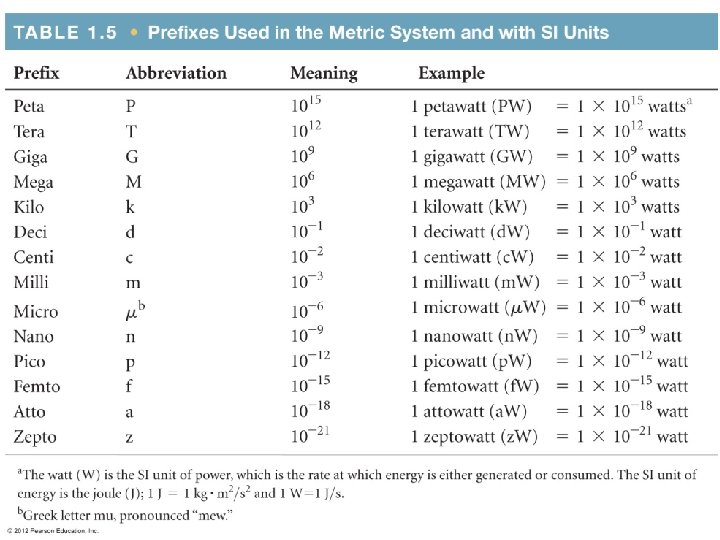



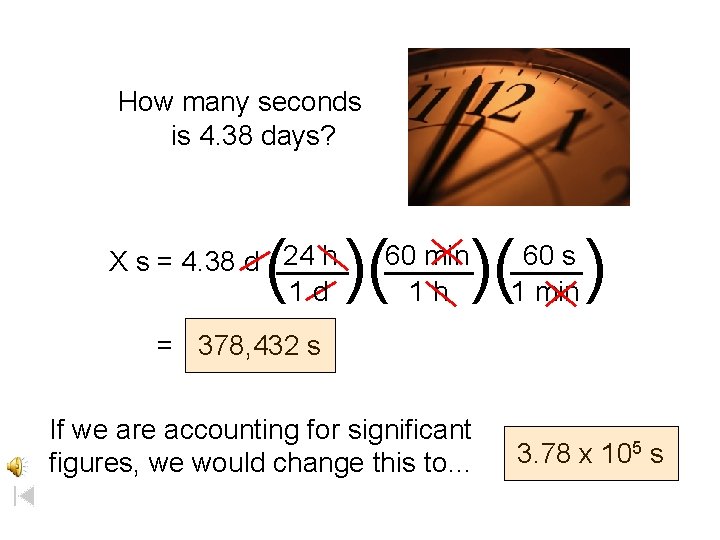
- Slides: 32

Conversion Factors and Unit Cancellation

A physical quantity must include: Number + Unit


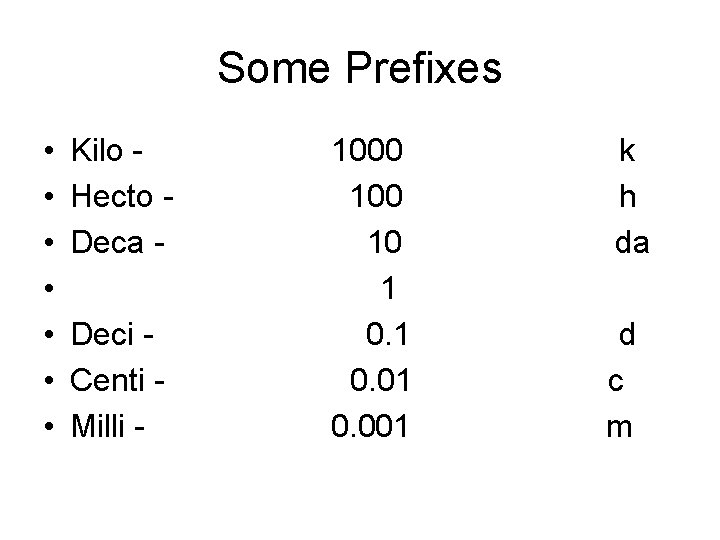
Some Prefixes • • Kilo - 1000 k Hecto - 100 h Deca - 10 da 1 Deci - 0. 1 d Centi - 0. 01 c Milli - 0. 001 m

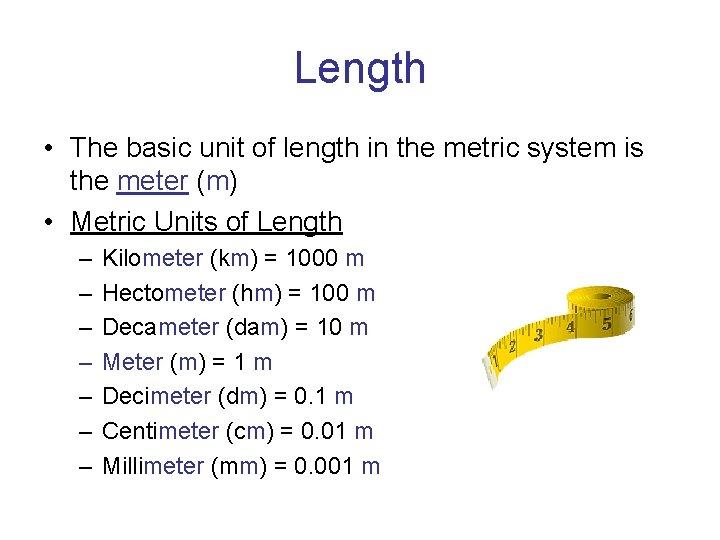
Length • The basic unit of length in the metric system is the meter (m) • Metric Units of Length – – – – Kilometer (km) = 1000 m Hectometer (hm) = 100 m Decameter (dam) = 10 m Meter (m) = 1 m Decimeter (dm) = 0. 1 m Centimeter (cm) = 0. 01 m Millimeter (mm) = 0. 001 m

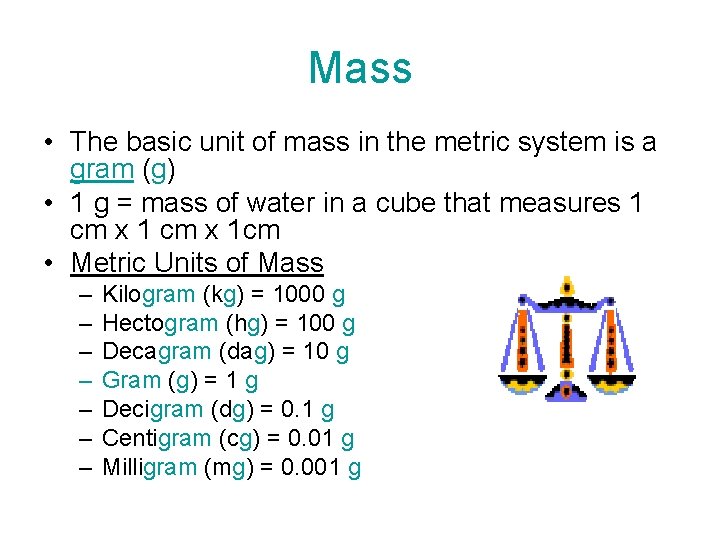
Mass • The basic unit of mass in the metric system is a gram (g) • 1 g = mass of water in a cube that measures 1 cm x 1 cm • Metric Units of Mass – – – – Kilogram (kg) = 1000 g Hectogram (hg) = 100 g Decagram (dag) = 10 g Gram (g) = 1 g Decigram (dg) = 0. 1 g Centigram (cg) = 0. 01 g Milligram (mg) = 0. 001 g

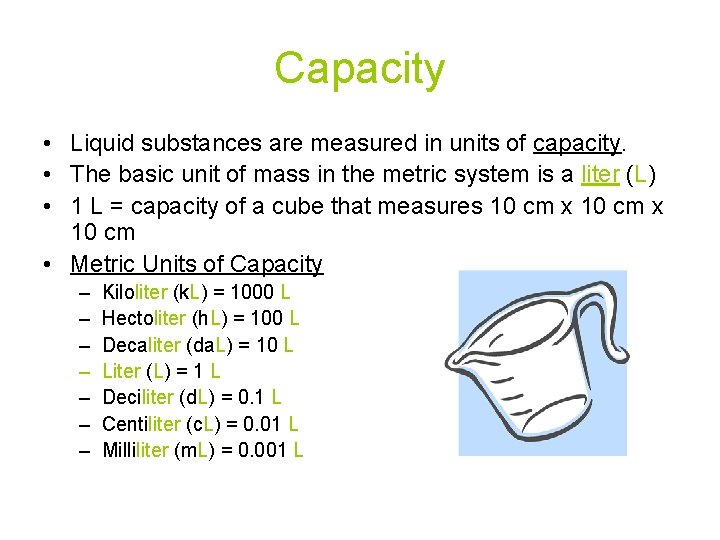
Capacity • Liquid substances are measured in units of capacity. • The basic unit of mass in the metric system is a liter (L) • 1 L = capacity of a cube that measures 10 cm x 10 cm • Metric Units of Capacity – – – – Kiloliter (k. L) = 1000 L Hectoliter (h. L) = 100 L Decaliter (da. L) = 10 L Liter (L) = 1 L Deciliter (d. L) = 0. 1 L Centiliter (c. L) = 0. 01 L Milliliter (m. L) = 0. 001 L

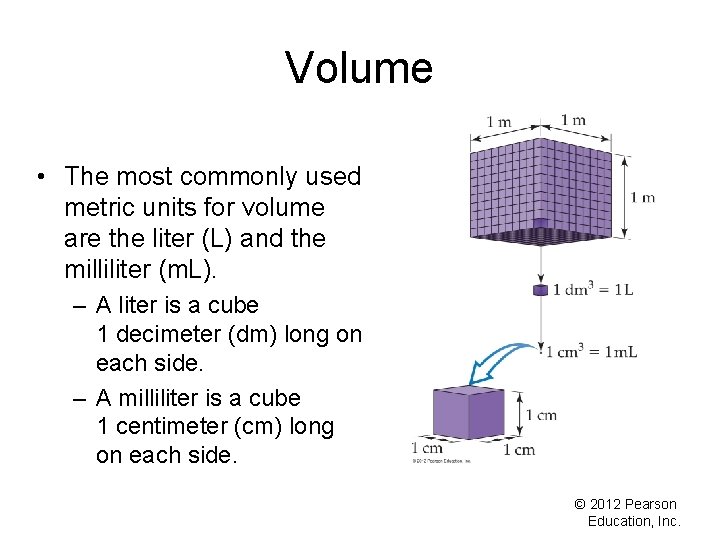
Volume • The most commonly used metric units for volume are the liter (L) and the milliliter (m. L). – A liter is a cube 1 decimeter (dm) long on each side. – A milliliter is a cube 1 centimeter (cm) long on each side. © 2012 Pearson Education, Inc.

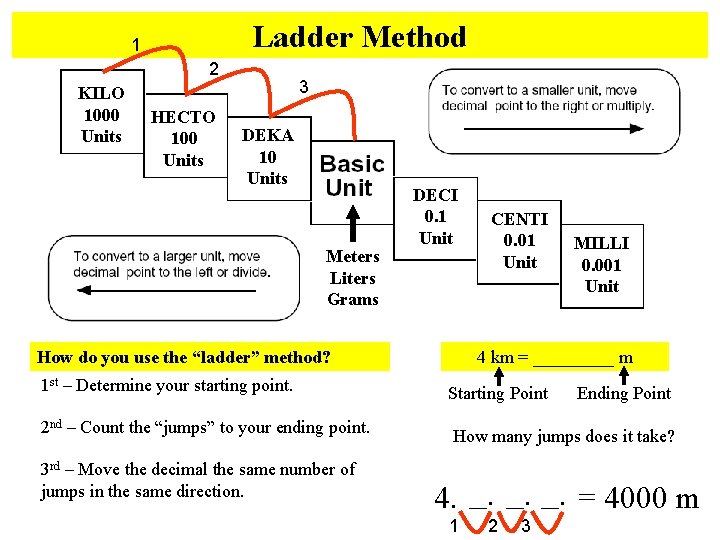
Ladder Method 1 2 KILO 1000 Units HECTO 100 Units 3 DEKA 10 Units Meters Liters Grams DECI 0. 1 Unit How do you use the “ladder” method? 1 st – Determine your starting point. 2 nd – Count the “jumps” to your ending point. 3 rd – Move the decimal the same number of jumps in the same direction. CENTI 0. 01 Unit MILLI 0. 001 Unit 4 km = _____ m Starting Point Ending Point How many jumps does it take? 4. __. __. = 4000 m 1 2 3

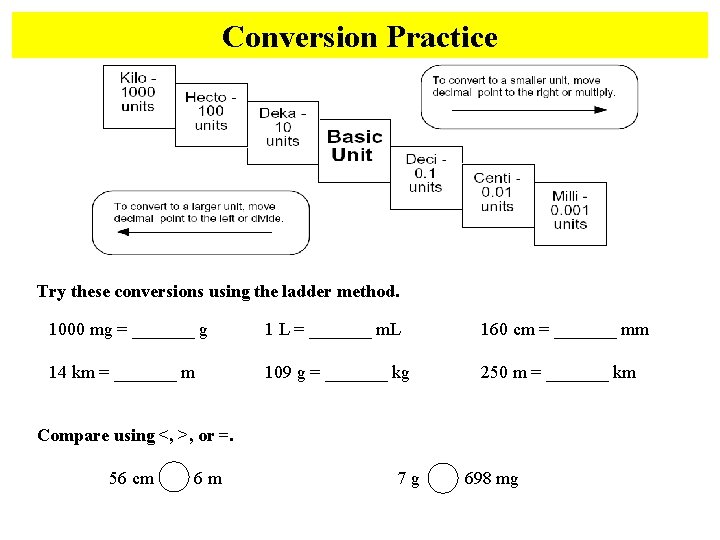
Conversion Practice Try these conversions using the ladder method. 1000 mg = _______ g 1 L = _______ m. L 160 cm = _______ mm 14 km = _______ m 109 g = _______ kg 250 m = _______ km Compare using <, >, or =. 56 cm 6 m 7 g 698 mg

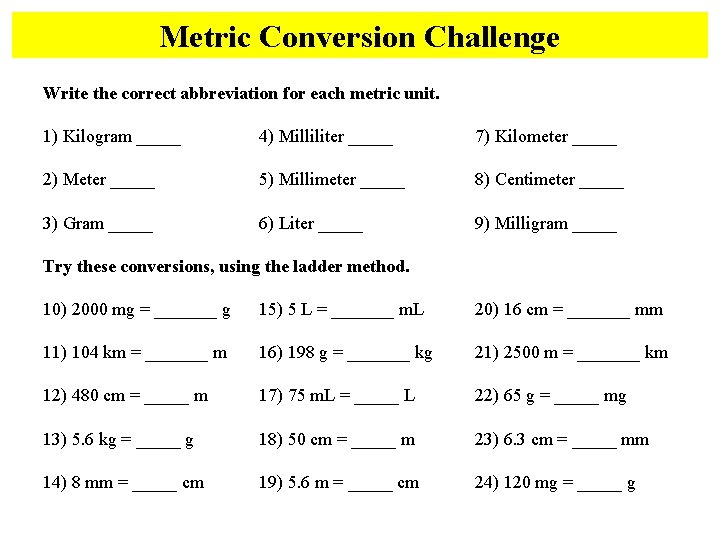
Metric Conversion Challenge Write the correct abbreviation for each metric unit. 1) Kilogram _____ 4) Milliliter _____ 7) Kilometer _____ 2) Meter _____ 5) Millimeter _____ 8) Centimeter _____ 3) Gram _____ 6) Liter _____ 9) Milligram _____ Try these conversions, using the ladder method. 10) 2000 mg = _______ g 15) 5 L = _______ m. L 20) 16 cm = _______ mm 11) 104 km = _______ m 16) 198 g = _______ kg 21) 2500 m = _______ km 12) 480 cm = _____ m 17) 75 m. L = _____ L 22) 65 g = _____ mg 13) 5. 6 kg = _____ g 18) 50 cm = _____ m 23) 6. 3 cm = _____ mm 14) 8 mm = _____ cm 19) 5. 6 m = _____ cm 24) 120 mg = _____ g

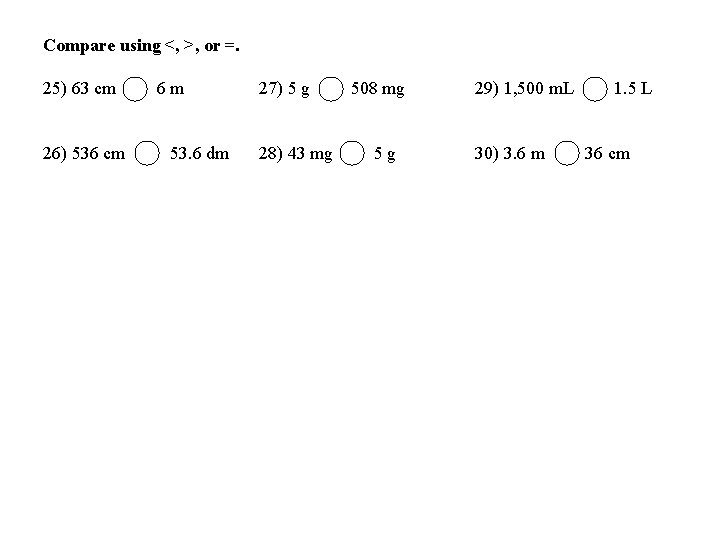
Compare using <, >, or =. 25) 63 cm 26) 536 cm 6 m 53. 6 dm 27) 5 g 28) 43 mg 508 mg 5 g 29) 1, 500 m. L 30) 3. 6 m 1. 5 L 36 cm

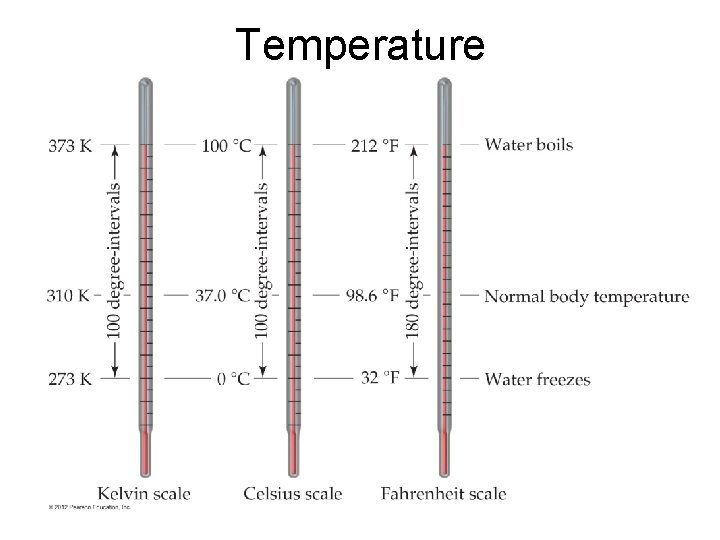
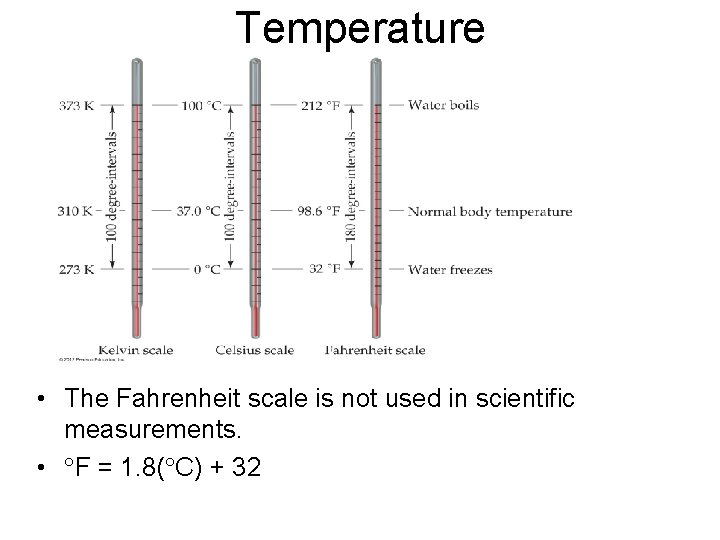
Temperature © 2012 Pearson Education, Inc.

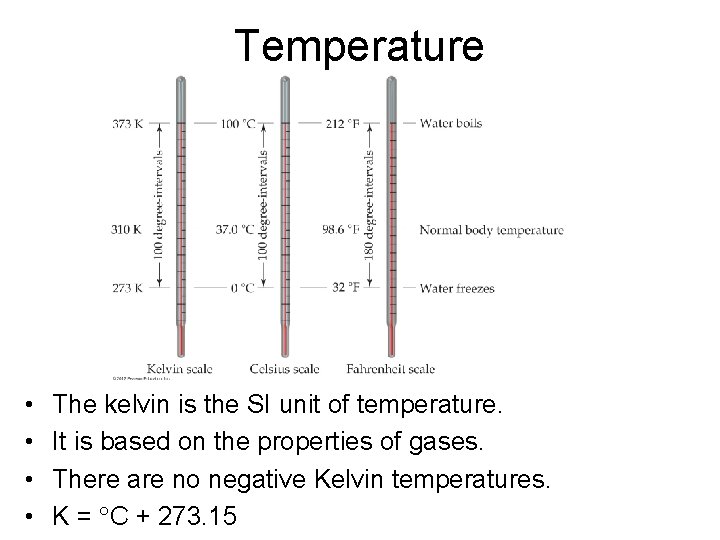
Temperature • • The kelvin is the SI unit of temperature. It is based on the properties of gases. There are no negative Kelvin temperatures. K = C + 273. 15

Temperature • The Fahrenheit scale is not used in scientific measurements. • F = 1. 8( C) + 32

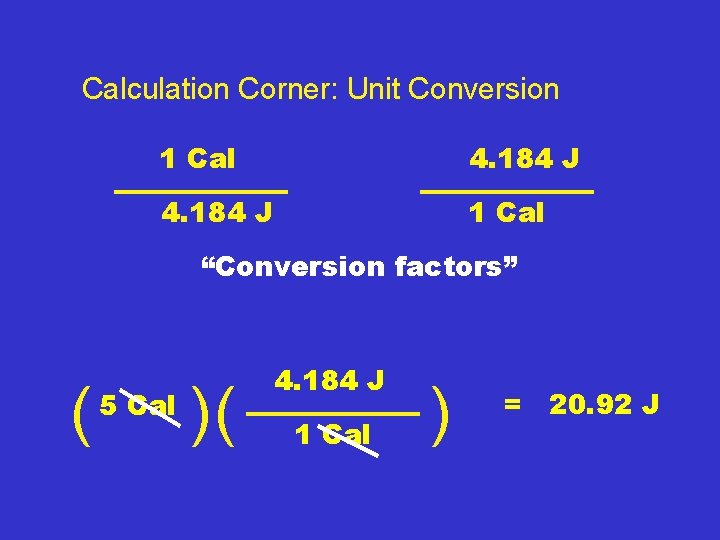
Calculation Corner: Unit Conversion 1 Cal = 4. 184 J

Calculation Corner: Unit Conversion 1 Cal 4. 184 J 1 Cal “Conversion factors”

Calculation Corner: Unit Conversion 1 Cal 4. 184 J 1 Cal “Conversion factors” ( 5 Cal )( 4. 184 J 1 Cal ) = 20. 92 J

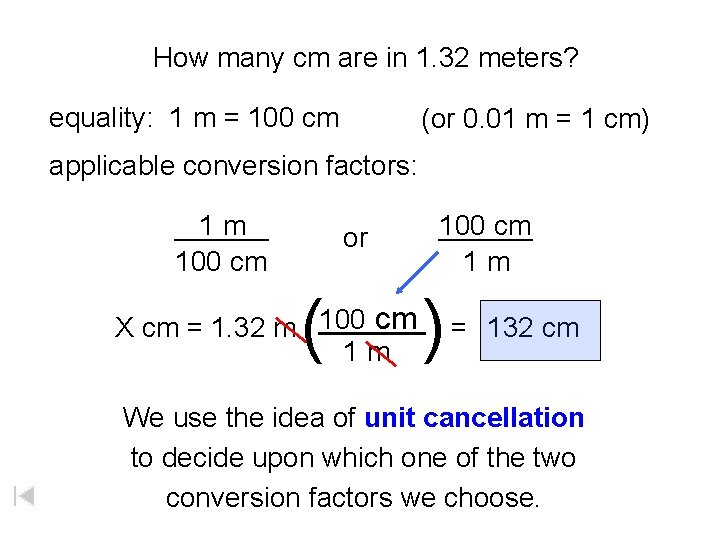
How many cm are in 1. 32 meters? equality: 1 m = 100 cm (or 0. 01 m = 1 cm) applicable conversion factors: ______ 1 m 100 cm or ( 100 cm ______ 1 m ) cm 100 ______ X cm = 1. 32 m = 132 cm 1 m We use the idea of unit cancellation to decide upon which one of the two conversion factors we choose.

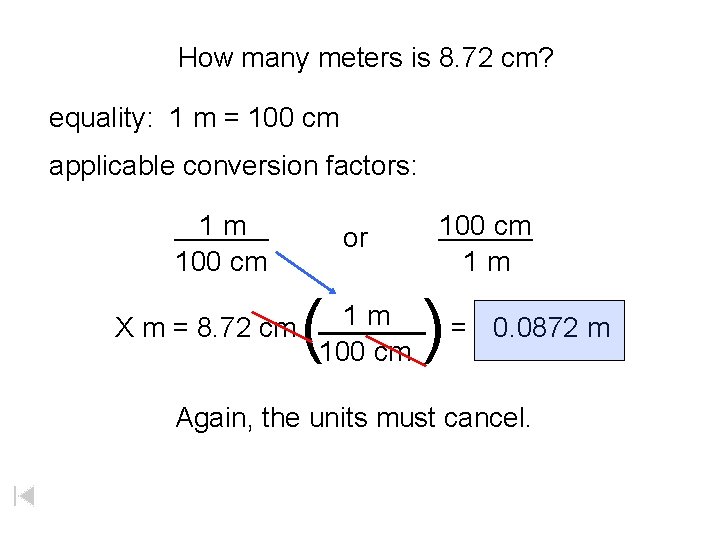
How many meters is 8. 72 cm? equality: 1 m = 100 cm applicable conversion factors: ______ 1 m 100 cm or ( 100 cm ______ 1 m ) 1 m ______ X m = 8. 72 cm = 0. 0872 m 100 cm Again, the units must cancel.

How many kilometers is 15, 000 decimeters? ( )( 1 m X km = 15, 000 dm ____ 10 dm ) 1 km ______ = 1. 5 km 1, 000 m
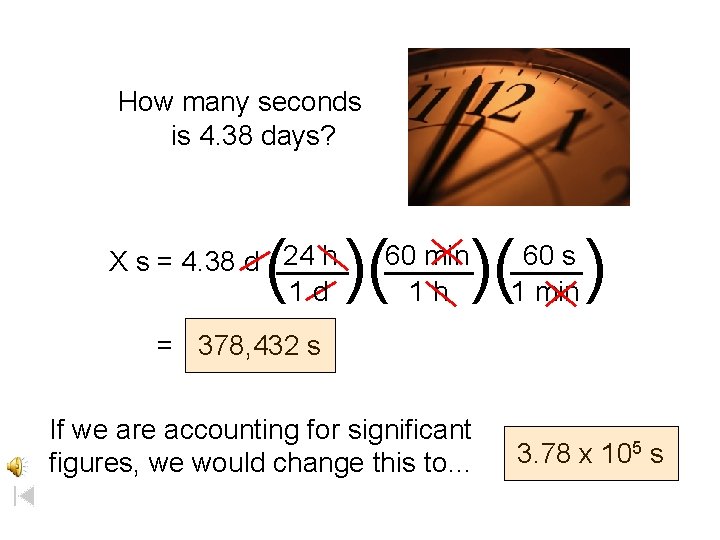
How many seconds is 4. 38 days? ( )( 24 h X s = 4. 38 d ____ 1 d )( ) 60 min _____ 1 h 60 s ____ 1 min = 378, 432 s If we are accounting for significant figures, we would change this to… 3. 78 x 105 s

Simple Math with Conversion Factors

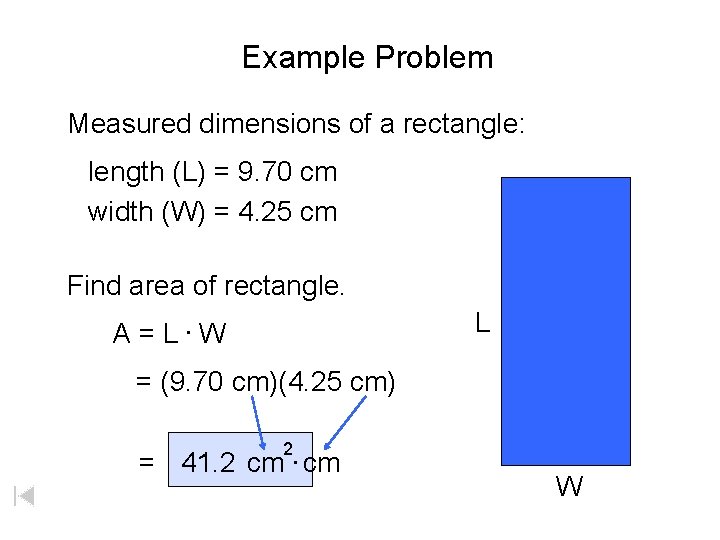
Example Problem Measured dimensions of a rectangle: length (L) = 9. 70 cm width (W) = 4. 25 cm Find area of rectangle. L A = L. W = (9. 70 cm)(4. 25 cm) = 41. 2 2 cm. cm W

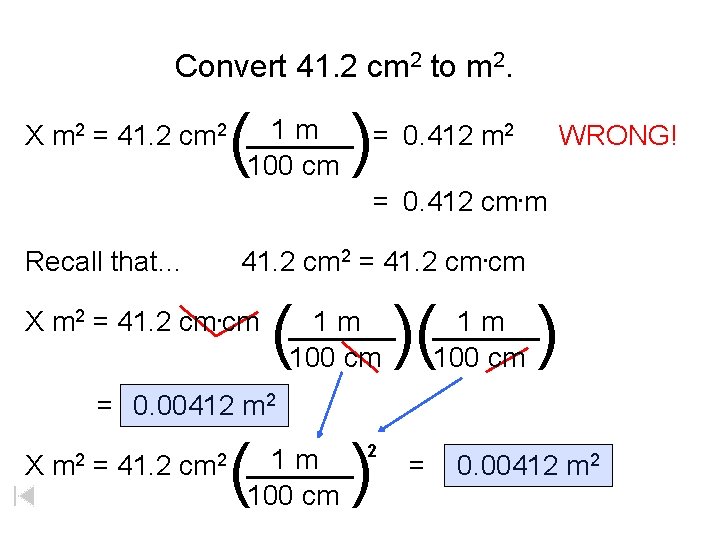
Convert 41. 2 cm 2 to m 2. ) ( 1 m X m 2 = 41. 2 cm 2 ______ = 0. 412 m 2 WRONG! 100 cm = 0. 412 cm. m Recall that… 41. 2 cm 2 = 41. 2 cm. cm X m 2 = 41. 2 cm. cm ( )( ) 1 m ______ 100 cm = 0. 00412 m 2 X m 2 = 41. 2 cm 2 ( ) 1 m ______ 100 cm 2 = 0. 00412 m 2

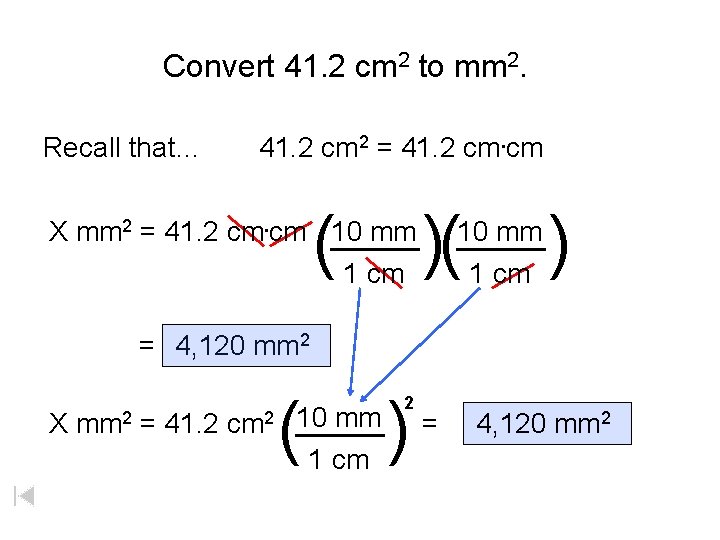
Convert 41. 2 cm 2 to mm 2. Recall that… 41. 2 cm 2 = 41. 2 cm. cm ( X mm 2 = 41. 2 cm. cm _____ 10 mm 1 cm )( 1 cm = 4, 120 mm 2 10 mm _____ ) = 4, 120 mm 2 ( 10 mm X mm 2 = 41. 2 cm 2 _____ 1 cm ) 2

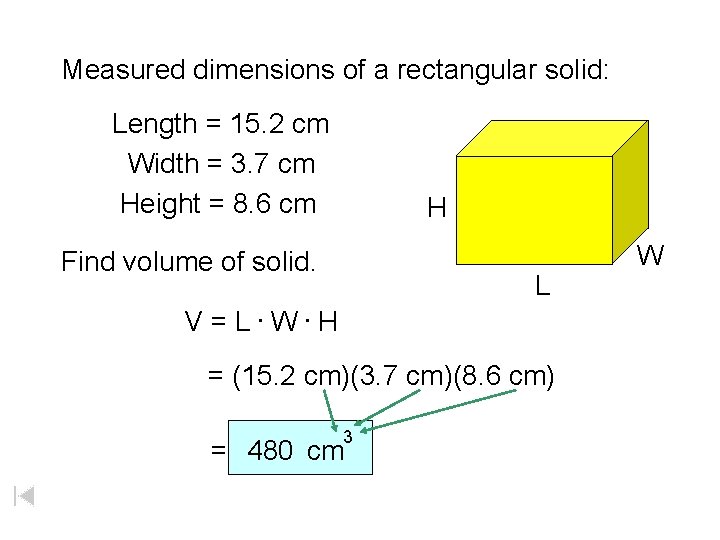
Measured dimensions of a rectangular solid: Length = 15. 2 cm Width = 3. 7 cm Height = 8. 6 cm H Find volume of solid. L V = L. W. H = (15. 2 cm)(3. 7 cm)(8. 6 cm) 3 = 480 cm W

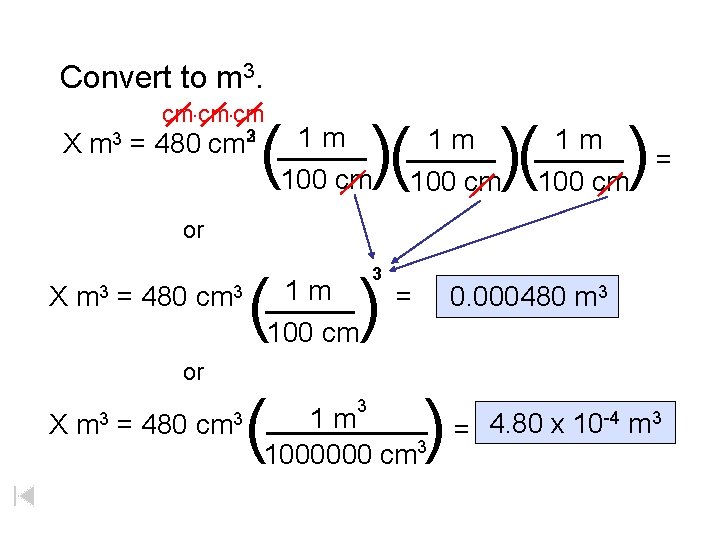
Convert to m 3. cm. cm ( )( 2 3 1 m X m 3 = 480 cm _____ 100 cm 1 m _____ )( 100 cm ) 1 m _____ = 100 cm or ( ) 1 m X m 3 = 480 cm 3 _____ 100 cm or X m 3 = 480 cm 3 ( 3 3 = 0. 000480 m 3 ) 1 m _____ 4. 80 x 10 -4 m 3 = 1000000 cm 3

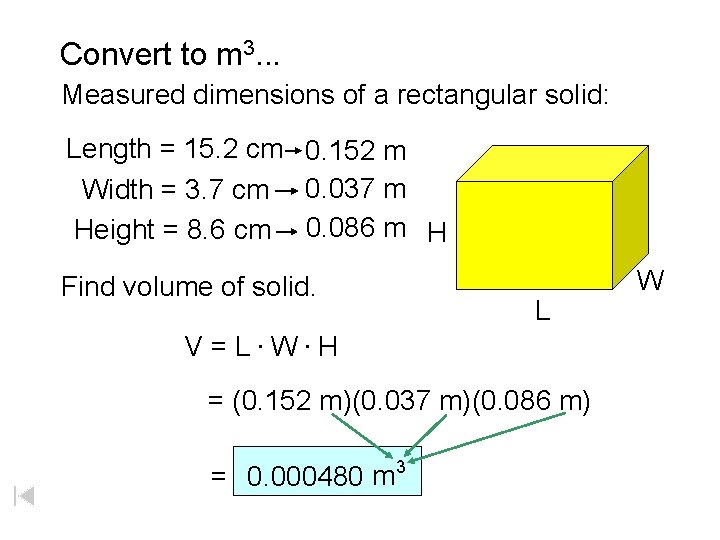
Convert to m 3. . . Measured dimensions of a rectangular solid: Length = 15. 2 cm 0. 152 m Width = 3. 7 cm 0. 037 m Height = 8. 6 cm 0. 086 m H Find volume of solid. L V = L. W. H = (0. 152 m)(0. 037 m)(0. 086 m) = 0. 000480 m 3 W

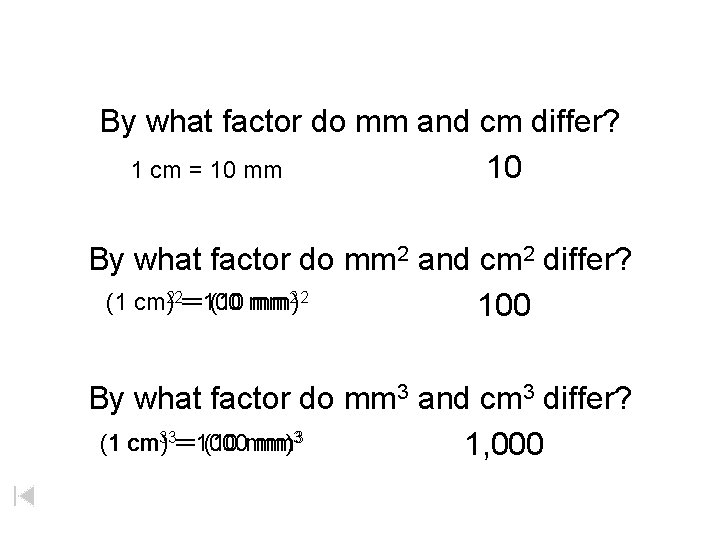
By what factor do mm and cm differ? 1 cm = 10 mm 10 By what factor do mm 2 and cm 2 differ? 1 cm 22 = 100 mm (1 cm) = (10 mm)2 2 100 By what factor do mm 3 and cm 3 differ? 1 cm 33 = 1000 mm (1 cm) = (10 mm)33 1, 000

