Concentration of Solutions Molarity Two solutions can contain






- Slides: 12

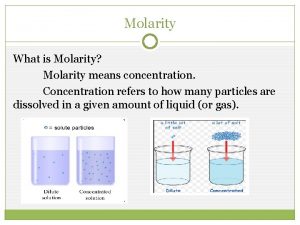
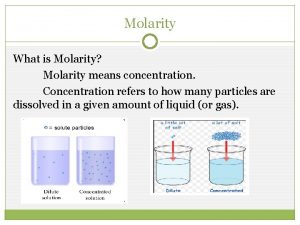
Concentration of Solutions

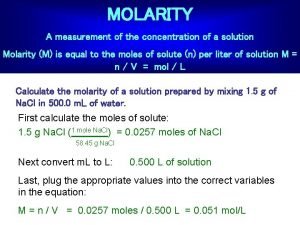
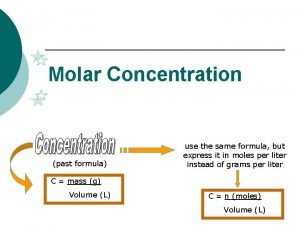
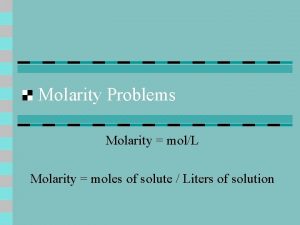
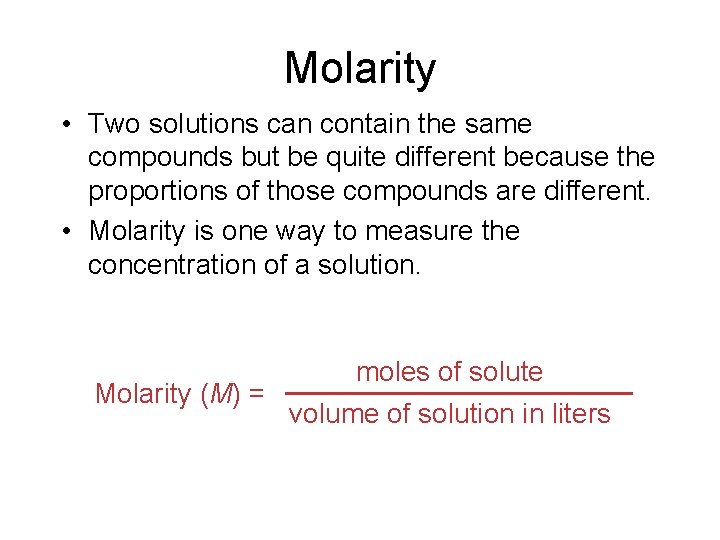
Molarity • Two solutions can contain the same compounds but be quite different because the proportions of those compounds are different. • Molarity is one way to measure the concentration of a solution. moles of solute Molarity (M) = volume of solution in liters

Mixing a Solution Procedure for preparing 0. 250 L of 1. 00 M solution of Cu. SO 4 • Weight out 39. 9 g of Cu. SO 4 = 0. 250 mol • Add 250 m. L of water to volumetric flask • Molarity =. 250 mol/. 250 L = 1. 00 M

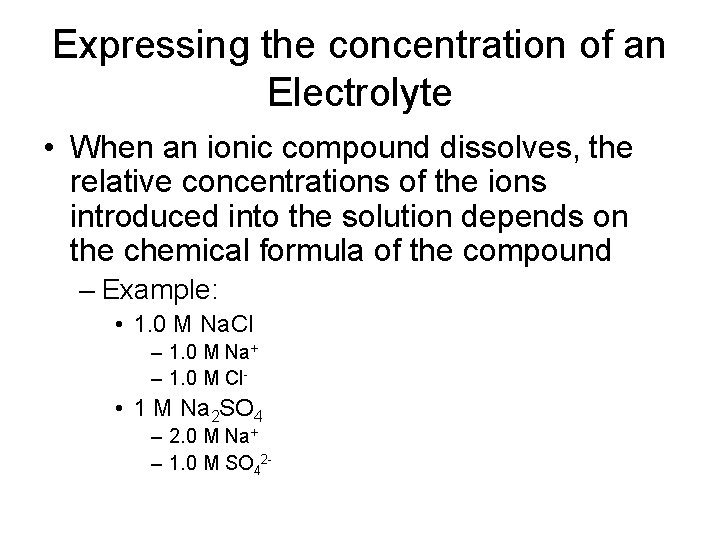
Expressing the concentration of an Electrolyte • When an ionic compound dissolves, the relative concentrations of the ions introduced into the solution depends on the chemical formula of the compound – Example: • 1. 0 M Na. Cl – 1. 0 M Na+ – 1. 0 M Cl- • 1 M Na 2 SO 4 – 2. 0 M Na+ – 1. 0 M SO 42 -

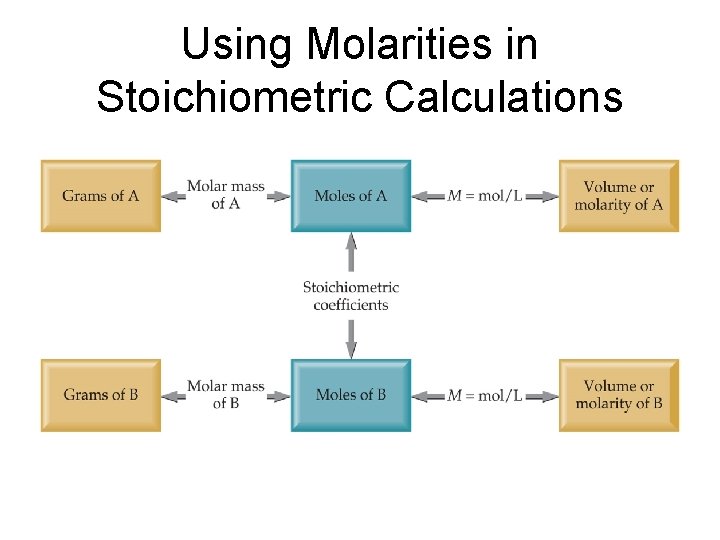
Using Molarities in Stoichiometric Calculations

Practice Question • How many moles of HNO 3 are in 2. 0 L of 0. 200 M HNO 3 solution? Moles = 2. 0 L soln x 0. 200 mol HNO 3 / 1 L solution =0. 40 mol HNO 3

Dilution

Dilution • Solutions of lower concentration can be obtained by adding water • Moles solute in conc soln = moles solute in dilution sln • Therefore, Mconc x Vconc = Mdil x Vdil

Practice problem • How many liters of 3. 0 M H 2 SO 4 are needed to make. 450 L of 0. 10 M H 2 SO 4? Mconc x Vconc = Mdil x Vdil Moles H 2 SO 4 in dilute solution = 0. 450 L x 0. 10 mol/L = 0. 045 mol = moles H 2 SO 4 in conc solution L conc soln = 0. 045 mol x 1 L/ 3. 0 mol =. 015 L

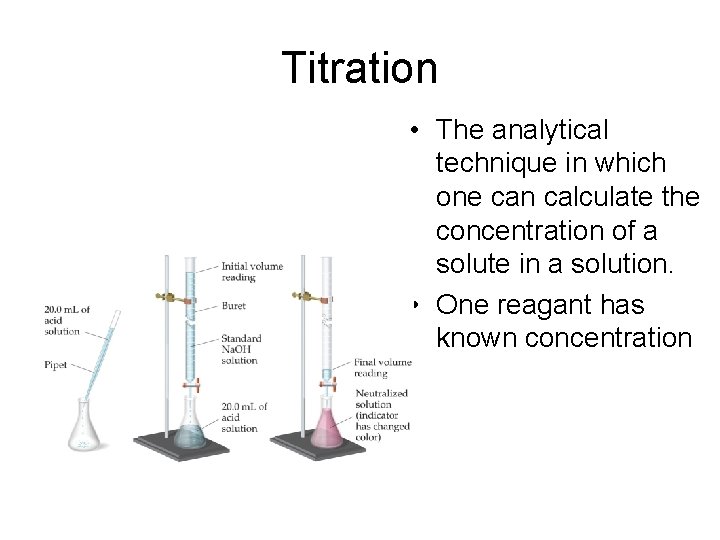
Titration • The analytical technique in which one can calculate the concentration of a solute in a solution. • One reagant has known concentration

Titration • Indicators are used to determine the equivalence point of the reaction – Point where the neutralization reaction between 2 reactants are complete – Reactants in stoichiometric equivalents are brought together

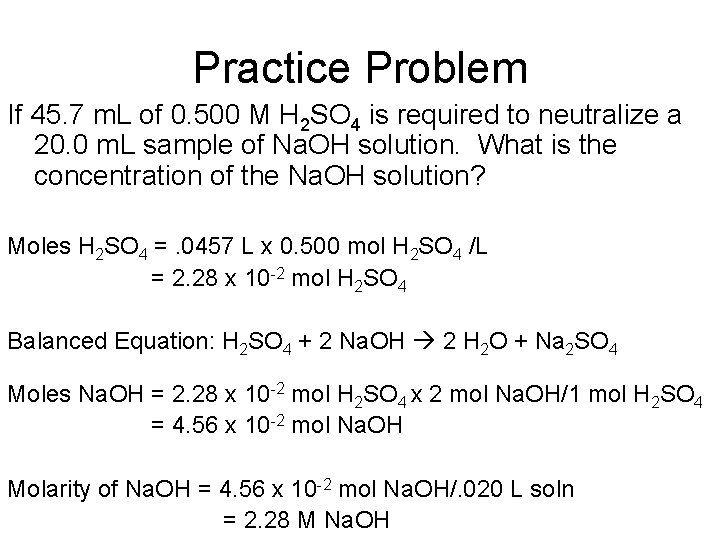
Practice Problem If 45. 7 m. L of 0. 500 M H 2 SO 4 is required to neutralize a 20. 0 m. L sample of Na. OH solution. What is the concentration of the Na. OH solution? Moles H 2 SO 4 =. 0457 L x 0. 500 mol H 2 SO 4 /L = 2. 28 x 10 -2 mol H 2 SO 4 Balanced Equation: H 2 SO 4 + 2 Na. OH 2 H 2 O + Na 2 SO 4 Moles Na. OH = 2. 28 x 10 -2 mol H 2 SO 4 x 2 mol Na. OH/1 mol H 2 SO 4 = 4. 56 x 10 -2 mol Na. OH Molarity of Na. OH = 4. 56 x 10 -2 mol Na. OH/. 020 L soln = 2. 28 M Na. OH
 Measures of concentration molarity quiz
Measures of concentration molarity quiz Molarity unit
Molarity unit What is molarity a measurement of?
What is molarity a measurement of? What is molarity a measurement of
What is molarity a measurement of How to find molecular concentration
How to find molecular concentration Molar concentration
Molar concentration Formula for molarity
Formula for molarity Molarity image
Molarity image Is concentration and molarity the same
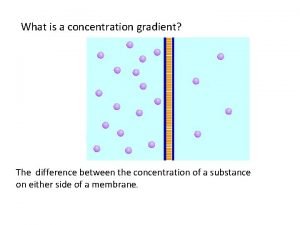
Is concentration and molarity the same Whats a concentration gradient
Whats a concentration gradient Movement of high concentration to low concentration
Movement of high concentration to low concentration Example of solution in science grade 7
Example of solution in science grade 7 Concentration of solutions
Concentration of solutions