Comparison and classification of methamphetamine seized in Japan













- Slides: 46

Comparison and classification of methamphetamine seized in Japan and Thailand using gas chromatography with liquid-liquid extraction and solid-phase microextraction Kenji Kuwayama et al.

2

INTRODUCTION 3

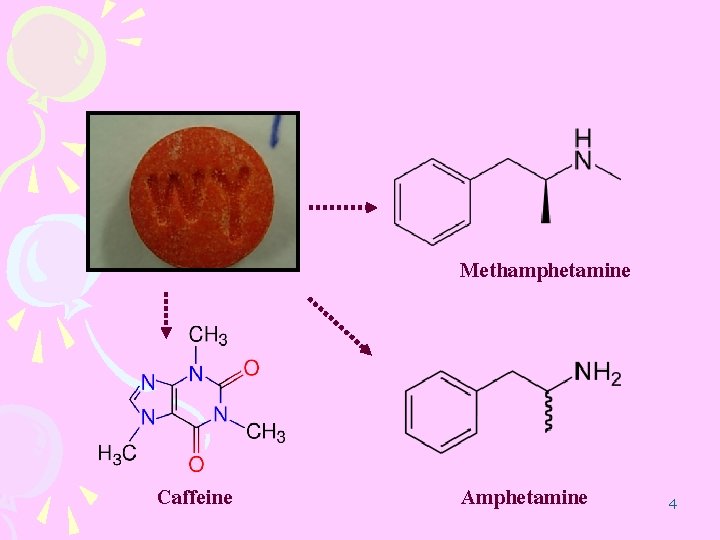
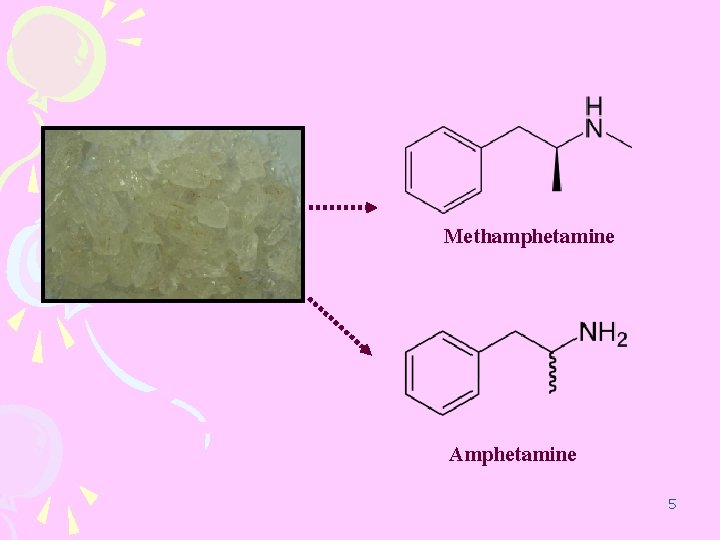
Methamphetamine Caffeine Amphetamine 4

Methamphetamine Amphetamine 5

eat inject smoke inhale 6

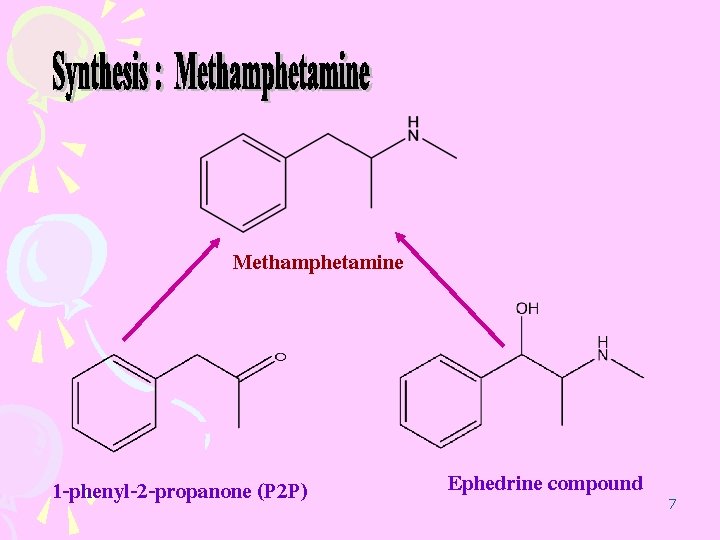
Methamphetamine 1 -phenyl-2 -propanone (P 2 P) Ephedrine compound 7

Presumptive Test Thin Layer Chromatography (TLC) Gas Chromatography Flame Ionization Detector (GC-FID) Gas Chromatography Mass Spectrometry (GC-MS) High Performance Liquid Chromatography (HPLC) Fourier Transform Infrared (FTIR) Spectroscopy ����� : United Nations Office on Drugs and Crime 8

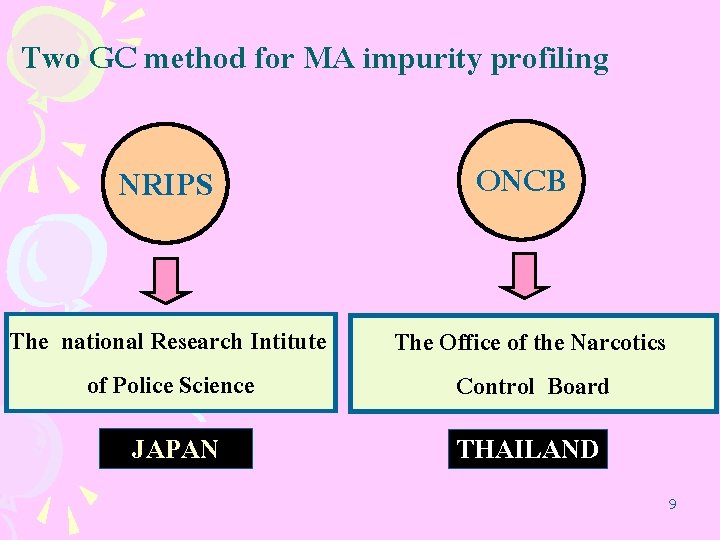
Two GC method for MA impurity profiling NRIPS ONCB The national Research Intitute of Police Science The Office of the Narcotics Control Board JAPAN THAILAND 9

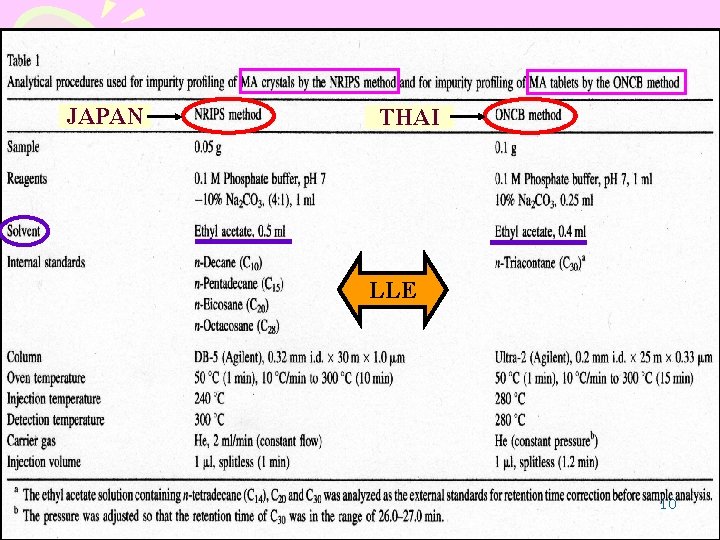
JAPAN THAI LLE 10

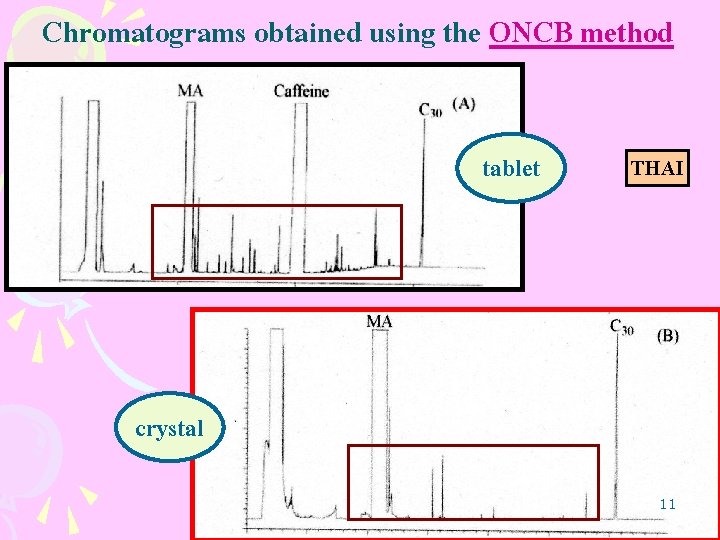
Chromatograms obtained using the ONCB method tablet THAI crystal 11

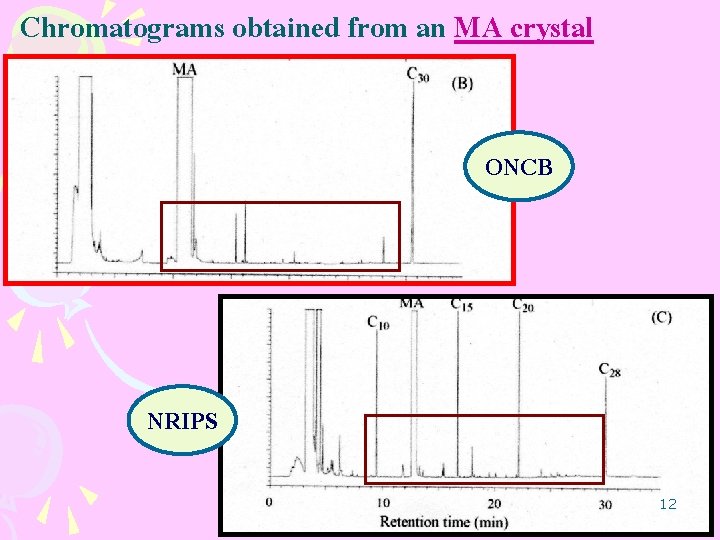
Chromatograms obtained from an MA crystal ONCB NRIPS 12

The aim of this study • Improve the analytical method for profiling MA impurity • Compare and classification MA crystals seized in different countries • Information in criminal investigation ; traffic routes , the source of supply and relationships between seizures 13

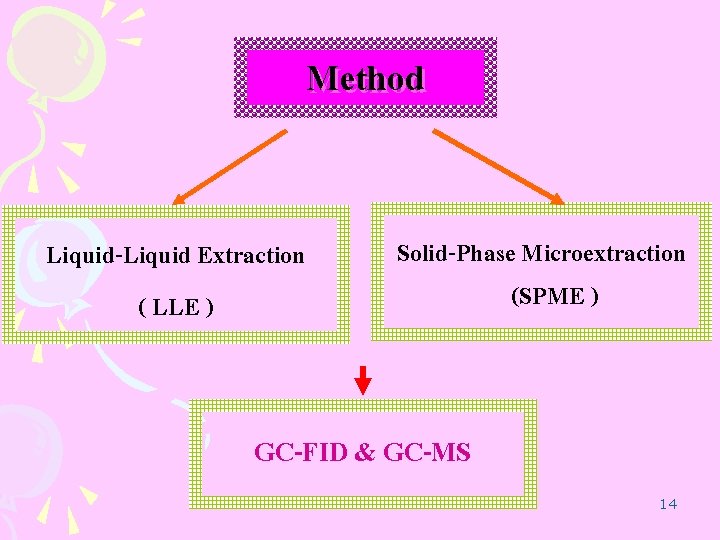
Method Liquid-Liquid Extraction ( LLE ) Solid-Phase Microextraction (SPME ) GC-FID & GC-MS 14

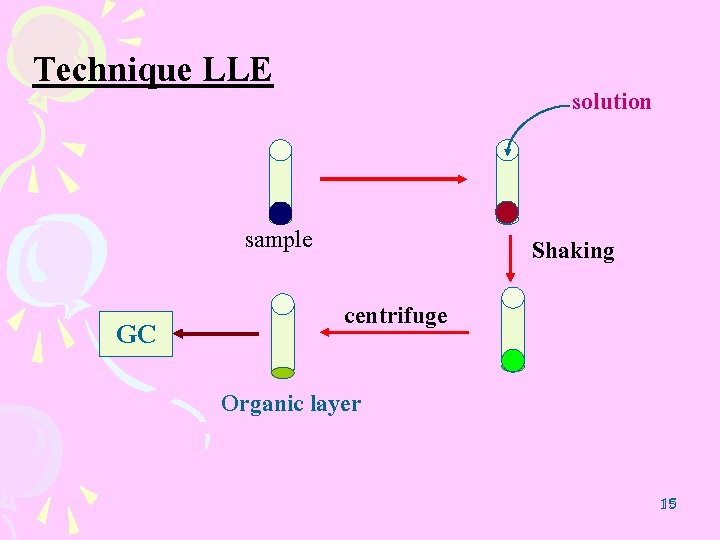
Technique LLE solution sample GC Shaking centrifuge Organic layer 15 15

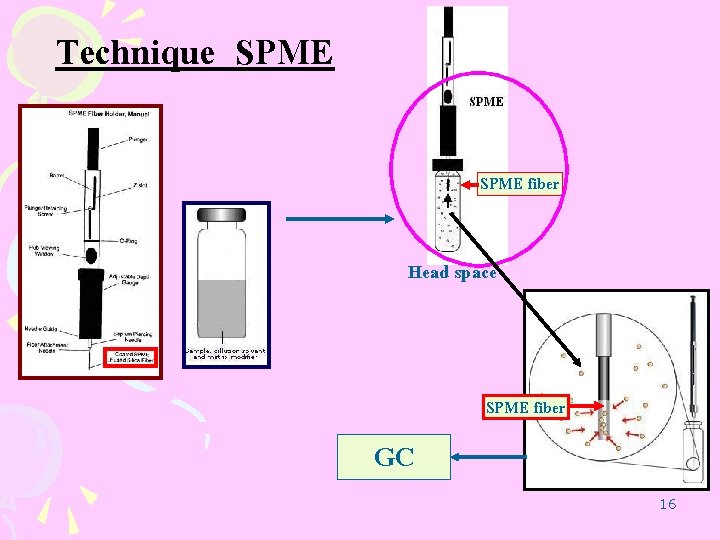
Technique SPME fiber Head space SPME fiber GC 16

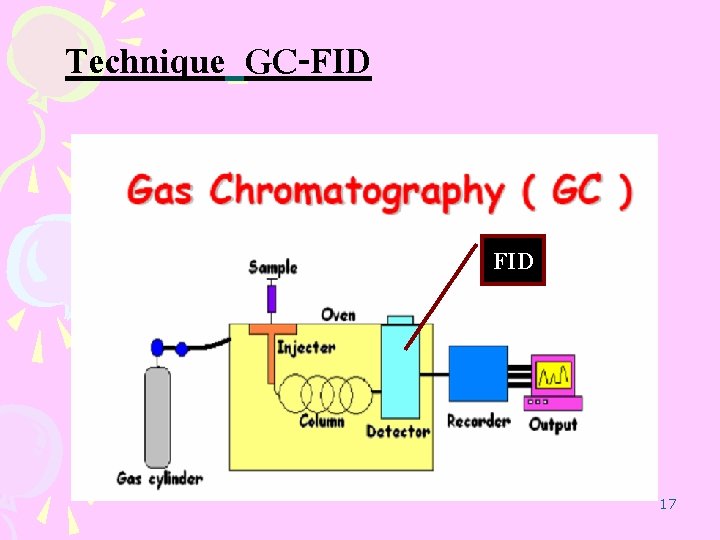
Technique GC-FID 17

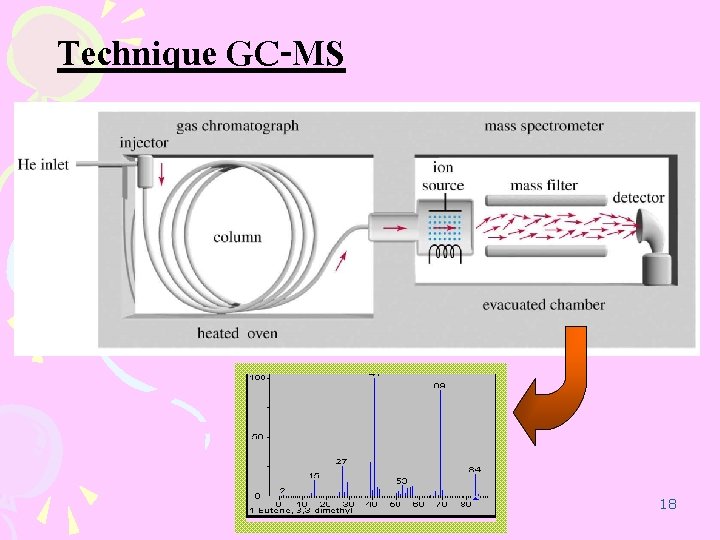
Technique GC-MS 18

MATERIALS AND METHODS 19

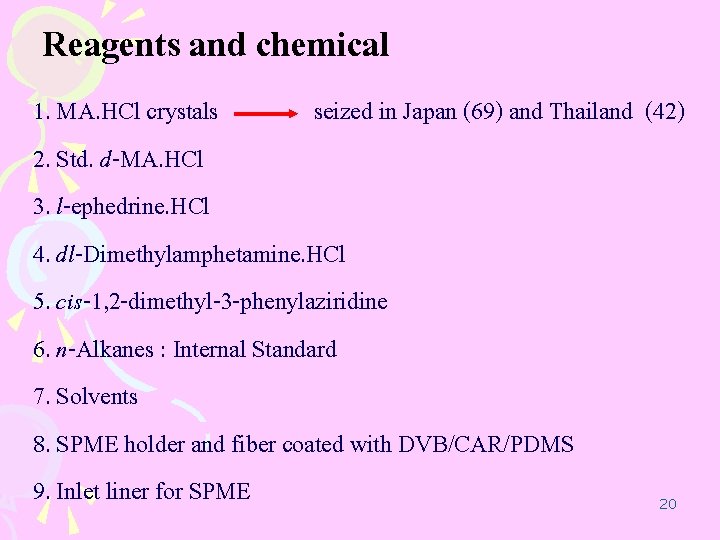
Reagents and chemical 1. MA. HCl crystals seized in Japan (69) and Thailand (42) 2. Std. d-MA. HCl 3. l-ephedrine. HCl 4. dl-Dimethylamphetamine. HCl 5. cis-1, 2 -dimethyl-3 -phenylaziridine 6. n-Alkanes : Internal Standard 7. Solvents 8. SPME holder and fiber coated with DVB/CAR/PDMS 9. Inlet liner for SPME 20

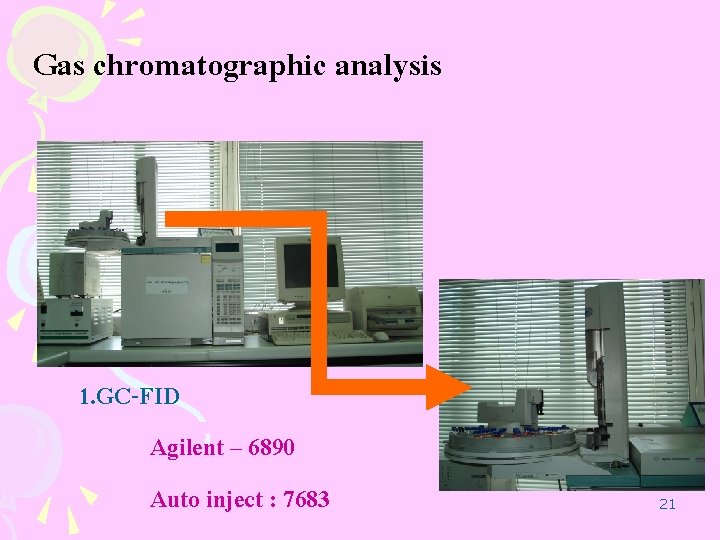
Gas chromatographic analysis 1. GC-FID Agilent – 6890 Auto inject : 7683 21

2. GC-MS Agilent – 6890 Agilent 5973 N MSD 22

COLUMN GC-FID & GC-MS DB-5 capillary column ( 30 m. x 0. 32 mm. x film thickness 1. 0 µm. ) 23

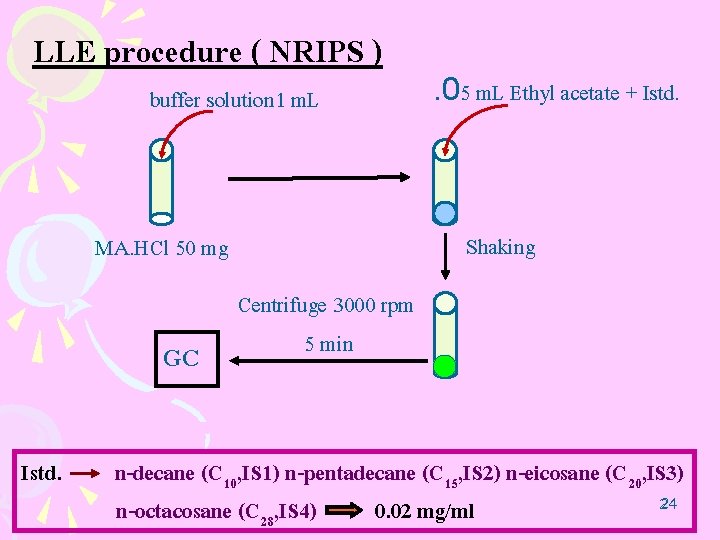
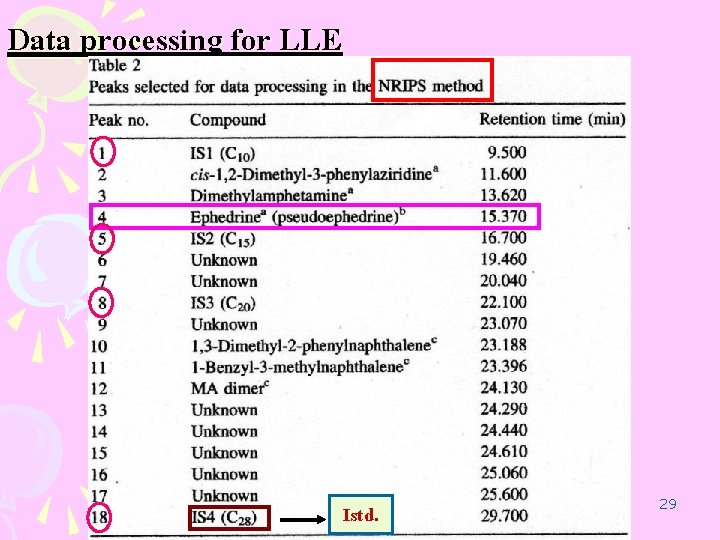
LLE procedure ( NRIPS ) buffer solution 1 m. L Shaking MA. HCl 50 mg GC Istd. . 05 m. L Ethyl acetate + Istd. Centrifuge 3000 rpm 5 min n-decane (C 10, IS 1) n-pentadecane (C 15, IS 2) n-eicosane (C 20, IS 3) 24 24 n-octacosane (C 28, IS 4) 0. 02 mg/ml

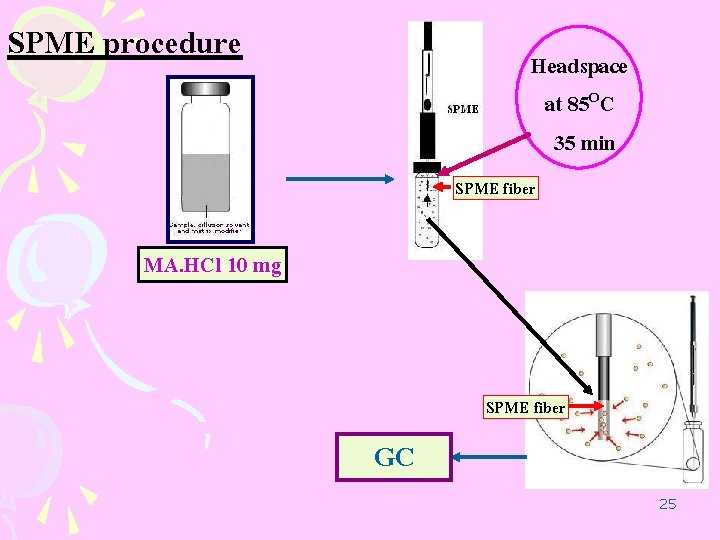
SPME procedure Headspace at 85 OC 35 min SPME fiber MA. HCl 10 mg SPME fiber GC 25

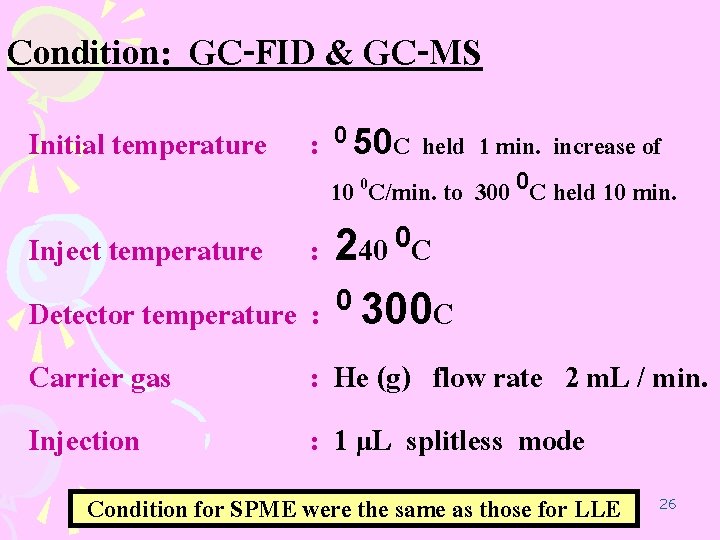
Condition: GC-FID & GC-MS Initial temperature : 0 50 C held 1 min. increase of 10 0 C/min. to 300 0 C held 10 min. 0 240 C Inject temperature : Detector temperature : 0 300 C Carrier gas : He (g) flow rate 2 m. L / min. Injection : 1 µL splitless mode Condition for SPME were the same as those for LLE 26

RESULTS 27

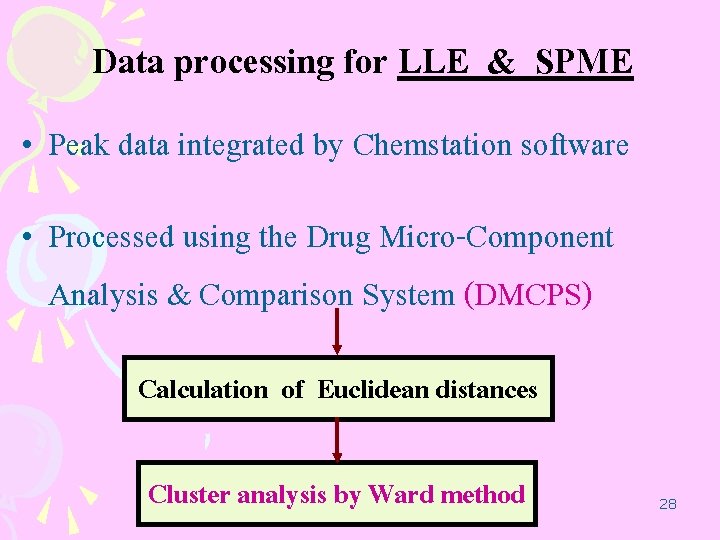
Data processing for LLE & SPME • Peak data integrated by Chemstation software • Processed using the Drug Micro-Component Analysis & Comparison System (DMCPS) Calculation of Euclidean distances Cluster analysis by Ward method 28

Data processing for LLE Istd. 29

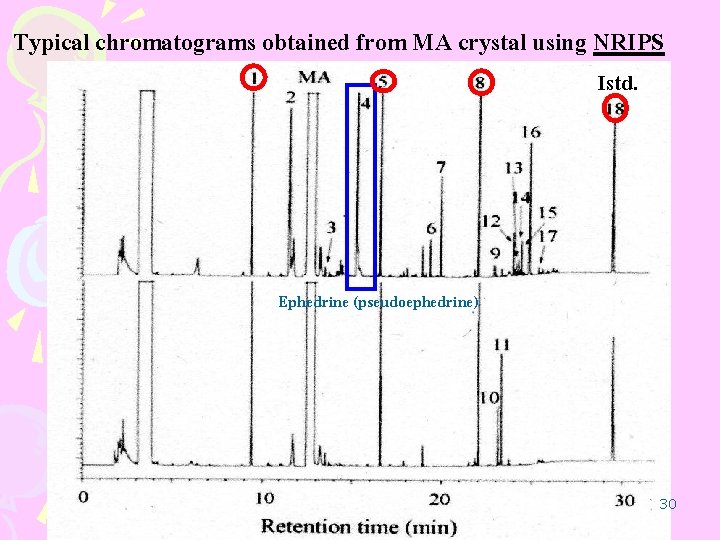
Typical chromatograms obtained from MA crystal using NRIPS Istd. Ephedrine (pseudoephedrine) 30

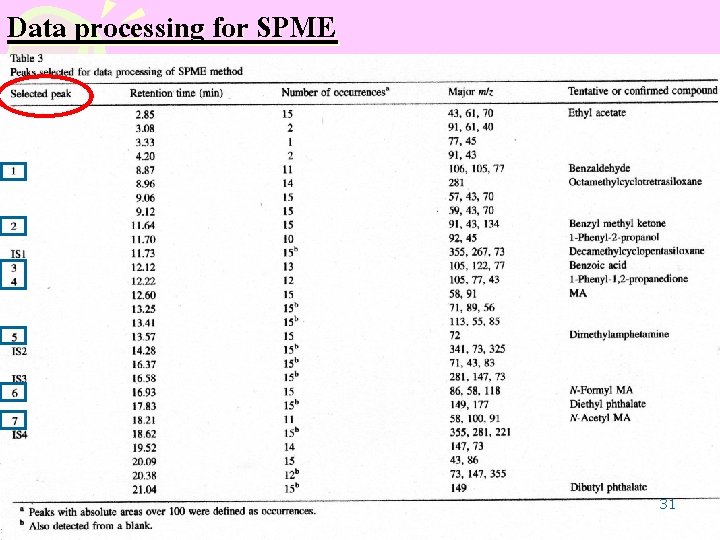
Data processing for SPME 31

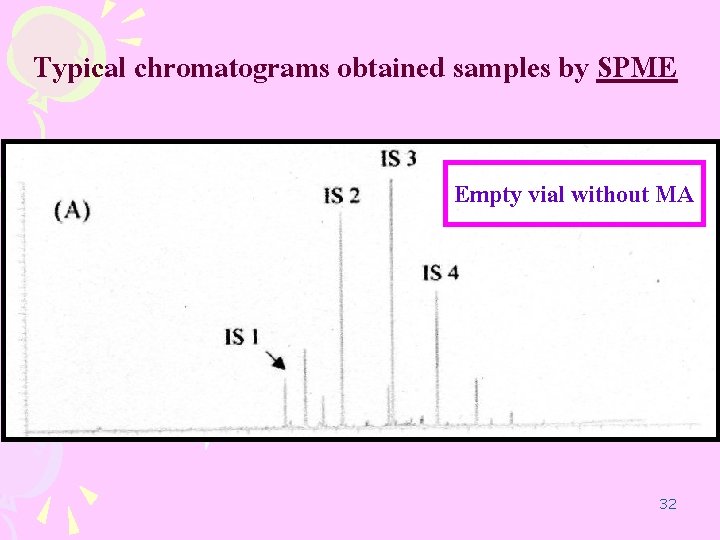
Typical chromatograms obtained samples by SPME Empty vial without MA 32

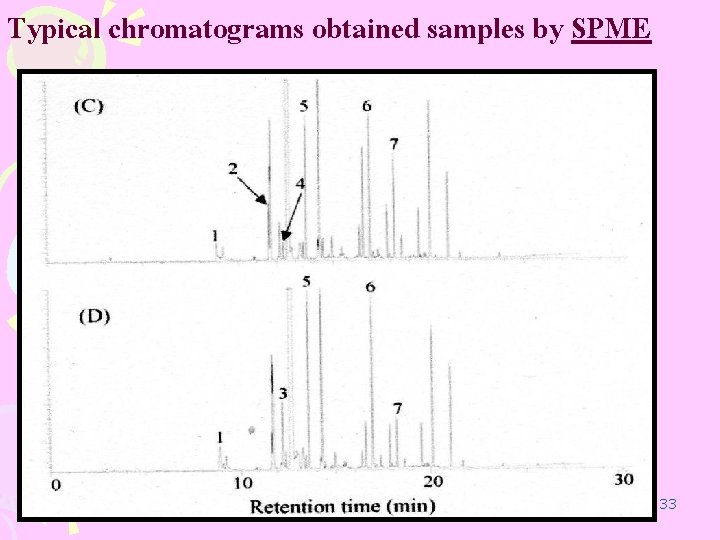
Typical chromatograms obtained samples by SPME 33

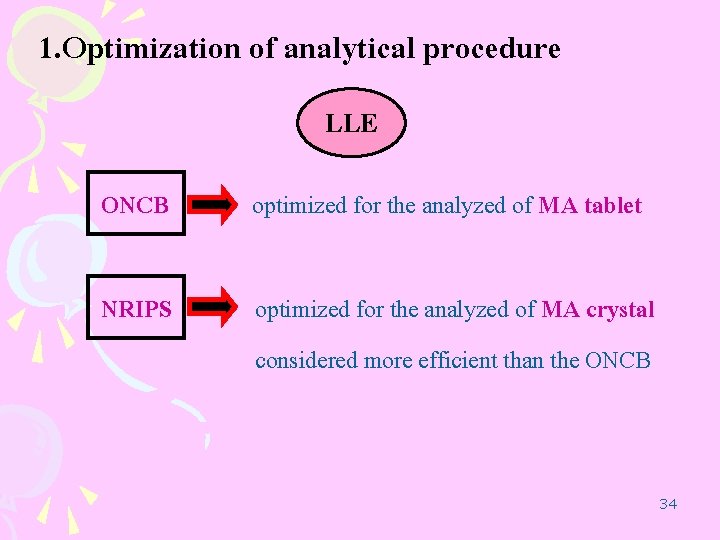
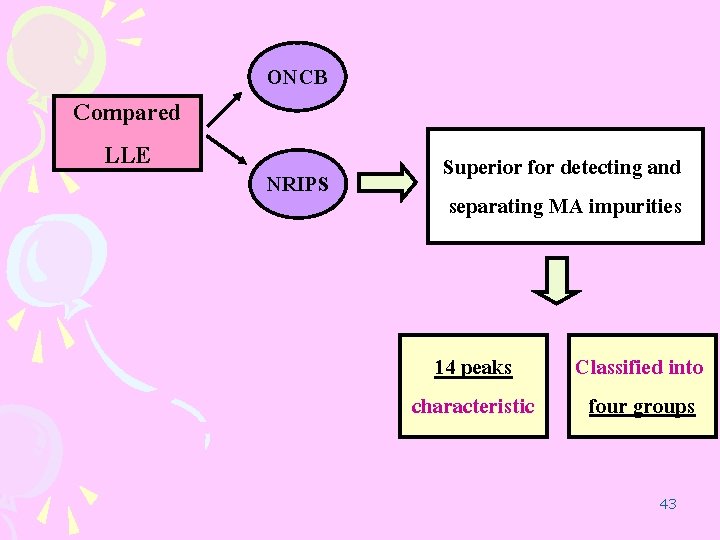
1. Optimization of analytical procedure LLE ONCB optimized for the analyzed of MA tablet NRIPS optimized for the analyzed of MA crystal considered more efficient than the ONCB 34

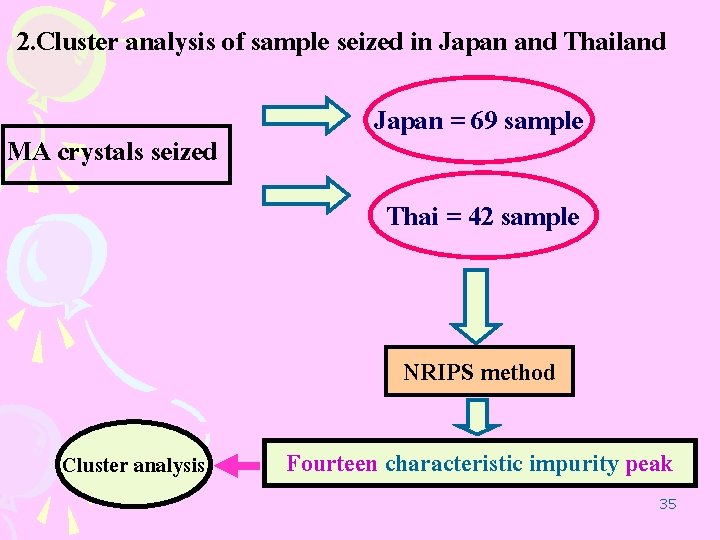
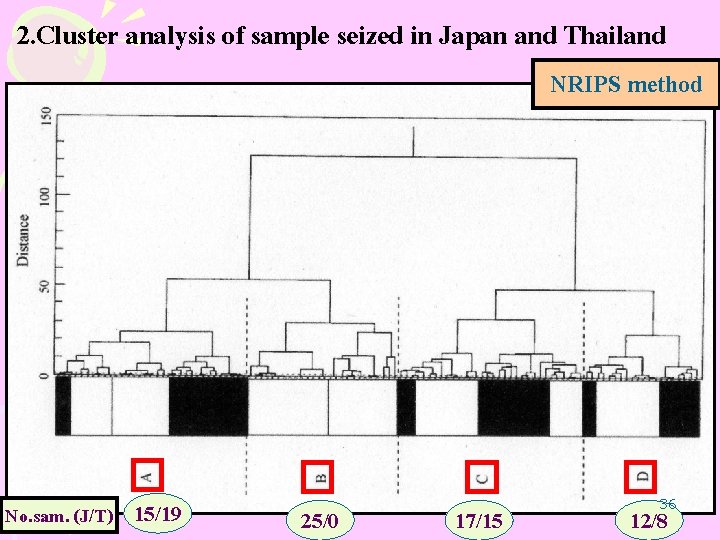
2. Cluster analysis of sample seized in Japan and Thailand MA crystals seized Japan = 69 sample Thai = 42 sample NRIPS method Cluster analysis Fourteen characteristic impurity peak 35

2. Cluster analysis of sample seized in Japan and Thailand NRIPS method No. sam. (J/T) 15/19 25/0 17/15 36 12/8

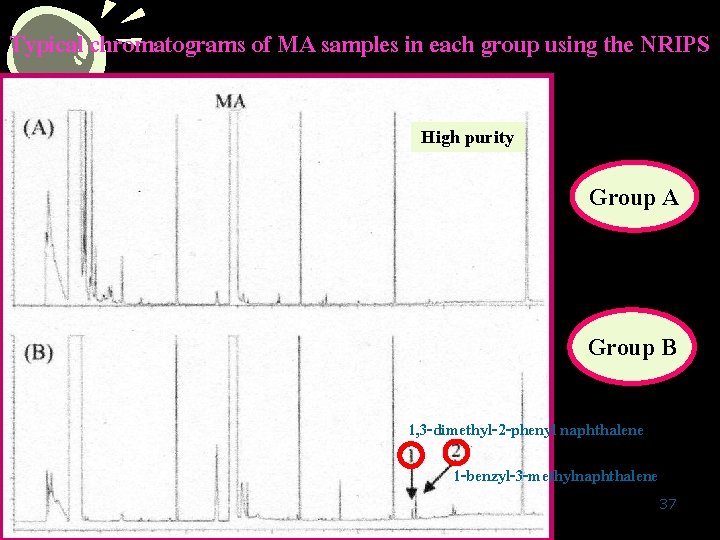
Typical chromatograms of MA samples in each group using the NRIPS High purity Group A Group B 1, 3 -dimethyl-2 -phenyl naphthalene 1 -benzyl-3 -methylnaphthalene 37

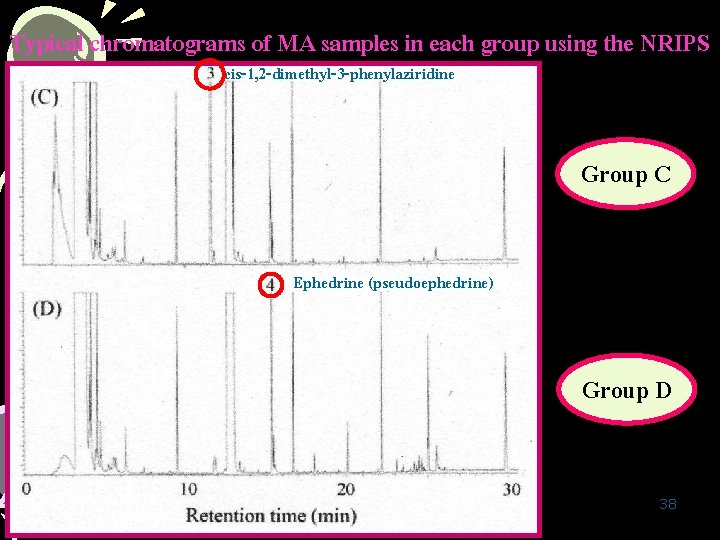
Typical chromatograms of MA samples in each group using the NRIPS cis-1, 2 -dimethyl-3 -phenylaziridine Group C Ephedrine (pseudoephedrine) Group D 38

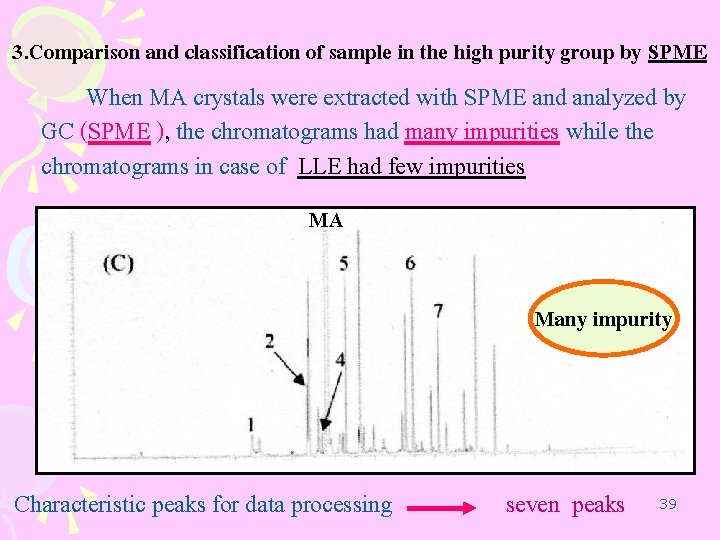
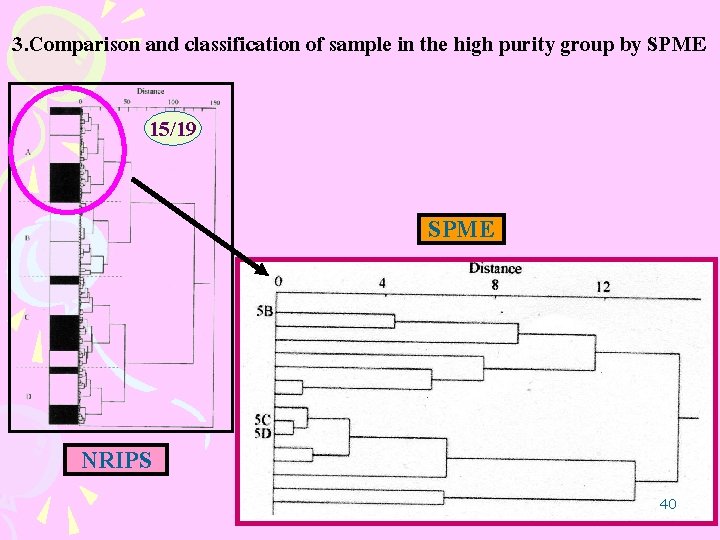
3. Comparison and classification of sample in the high purity group by SPME When MA crystals were extracted with SPME and analyzed by GC (SPME ), the chromatograms had many impurities while the chromatograms in case of LLE had few impurities MA Many impurity Characteristic peaks for data processing seven peaks 39

3. Comparison and classification of sample in the high purity group by SPME 15/19 SPME NRIPS 40

3. Comparison and classification of sample in the high purity group by SPME Chromatograms were distinguished clearly from SPME method , whereas in the case of LLE it was difficult to compare and classify samples in the highpurity group because there were so few impurities. The SPME method enables us to compare and classify high-purity MA 41

CONCLUSION 42

Compared LLE ONCB NRIPS Superior for detecting and separating MA impurities 14 peaks characteristic Classified into four groups 43

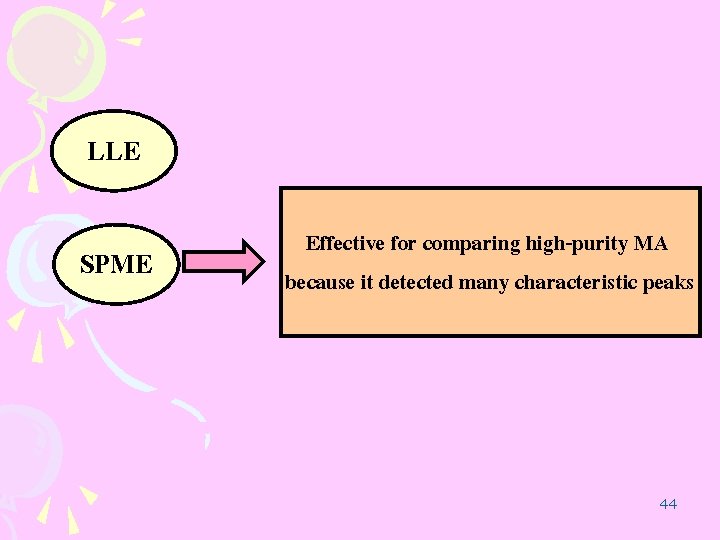
LLE SPME Effective for comparing high-purity MA because it detected many characteristic peaks 44

45

QUESTION 46
 Dr eugene barone
Dr eugene barone Methamphetamine addict
Methamphetamine addict Methamphetamine other names
Methamphetamine other names Panic seized the writer passive voice
Panic seized the writer passive voice No temptation has seized you
No temptation has seized you No temptation has seized you
No temptation has seized you Panic seized the writer passive voice
Panic seized the writer passive voice Scientific working group for the analysis of seized drugs
Scientific working group for the analysis of seized drugs Limit convergence test
Limit convergence test Qualitative classification in statistics
Qualitative classification in statistics Comparison chart romeo and juliet vs. west side story
Comparison chart romeo and juliet vs. west side story Definition of compare and contrast essay
Definition of compare and contrast essay Comparison and critique of osi and tcp/ip model
Comparison and critique of osi and tcp/ip model Comparison and critique of osi and tcp/ip model
Comparison and critique of osi and tcp/ip model How are the physical features of japan and korea similar?
How are the physical features of japan and korea similar? Past present future in japanese
Past present future in japanese The age of exploration outcome china and japan's reactions
The age of exploration outcome china and japan's reactions Japan during the age of exploration
Japan during the age of exploration Crime and punishment in medieval japan
Crime and punishment in medieval japan Spread of china's literature to heian japan and korea
Spread of china's literature to heian japan and korea Ministry of land infrastructure transport and tourism
Ministry of land infrastructure transport and tourism Europe and japan in ruins
Europe and japan in ruins Germany and italy
Germany and italy Knights vs samurai venn diagram
Knights vs samurai venn diagram Lazy learning and eager learning
Lazy learning and eager learning Traditional classification vs modern classification
Traditional classification vs modern classification Block method outline
Block method outline Comparison contrast paragraph
Comparison contrast paragraph Conservancy system
Conservancy system Datagram vs virtual circuit
Datagram vs virtual circuit Inter firm comparison images
Inter firm comparison images Comparing kamikaze and the prelude
Comparing kamikaze and the prelude Emigree structure
Emigree structure Parents and offspring
Parents and offspring Comparison between monitoring and evaluation
Comparison between monitoring and evaluation Description as pattern of paragraph development
Description as pattern of paragraph development Patterns in development in writing
Patterns in development in writing Sarcoplasmic
Sarcoplasmic The network layer is concerned with of data.
The network layer is concerned with of data. Characteristics of skeletal smooth and cardiac muscle
Characteristics of skeletal smooth and cardiac muscle Purposes of micro teaching
Purposes of micro teaching Ozymandias and london venn diagram
Ozymandias and london venn diagram Comparison of endocrine and nervous system
Comparison of endocrine and nervous system Earth and moon size comparison
Earth and moon size comparison Pin diagram of 80386 microprocessor
Pin diagram of 80386 microprocessor What is the difference between real self and ideal self?
What is the difference between real self and ideal self? Differences between joyce and woolf
Differences between joyce and woolf