Companys Presentation August 2011 Disclaimer This presentation has













- Slides: 41

Company’s Presentation August 2011

Disclaimer This presentation has been prepared by DIOD OJSC (the "Company“, “Group” or “DIOD”). By attending the meeting where the presentation is made, or by reading the presentation, you agree to the limitations and notifications set out below. This presentation is strictly confidential to the recipient and may not be reproduced or redistributed, passed on, or the contents otherwise divulged, directly or indirectly, to any other person or published in whole or in part for any purpose. Failure to comply with this restriction may constitute a violation of applicable securities laws. This presentation has been prepared solely for your information in connection with the possible offering of securities of the Company. The presentation does not constitute or form part of, and should not be construed as, an offer, solicitation or invitation to subscribe for, underwrite or otherwise acquire, any securities of the Company or any member of its group nor should it or any part of it form the basis of, or be relied on in connection with, any contract to purchase or subscribe for any securities of the Company or any member of its group, nor shall it or any part of it form the basis of or be relied on in connection with any contract or commitment whatsoever. Any person considering the purchase of any securities of the Company must inform himself or herself independently based solely on the Company's prospectus and any amendments or supplements thereto before taking any investment decision. The prospectus, when issued, may contain information different from the information contained in this presentation. This presentation includes forward-looking statements. These forward-looking statements include statements concerning targets, plans, objectives, goals, strategies, future events or performance, and underlying assumptions and other statements, which are other than statements of historical facts. The words "believe", "expect", "anticipate", "intend", "estimate", "forecast", "project", "will", "may", "should" and similar expressions identify forwardlooking statements. Forward-looking statements include statements regarding: strategies, outlook and growth prospects; future plans and potential for future growth; liquidity, capital resources and capital expenditures; growth in demand for products; economic outlook and industry trends; developments of markets; the impact of regulatory initiatives; and the strength of competitors. By their nature, forward-looking statements involve risks and uncertainties, because they relate to events and depend on circumstances that may or may not occur in the future. The forward-looking statements in this presentation are based upon various assumptions, many of which are based, in turn, upon further assumptions, including, without limitation, data available from third parties. Although the Company believes that these assumptions were reasonable when made, these assumptions are inherently subject to significant uncertainties and contingencies which are difficult or impossible to predict and are beyond the Company's control, and the Company may not achieve or accomplish these targets, expectations, beliefs or projections. The Company cautions you that forward-looking statements are not guarantees of future performance and that its actual results of operations, financial condition and liquidity and the development of the industry in which the Company operates may differ materially from those made in or suggested by the forwardlooking statements contained in this presentation. These forward looking statements speak only as at the date as of which they are made and, neither the Company, nor any of its agents, employees or advisors intend or have any duty or obligation to supplement, amend, update or revise any of the forward-looking statements contained in this presentation to reflect any change in the Company's expectations with regard thereto or any change in events, conditions, or circumstances on which any such statements are based. The Company does not undertake any obligation to review or confirm analysts' expectations. This presentation is not directed to or intended for distribution to or use by, any person or entity that is a citizen or resident or located in any locality, state, country or other jurisdiction where such distribution, publication, availability or use would be contrary to law or regulation or which would require any registration or licensing within such jurisdiction. This presentation is not an offer or solicitation to purchase or subscribe for securities in the United States. The securities of the Company have not been registered under the US Securities Act of 1933, as amended (the "Securities Act") and may not be offered or sold in the United States or to or for the account or benefit of US persons (as such terms are defined in Regulation S under the Securities Act) unless registered under the Securities Act or pursuant to an exemption from such registration. The Company does not intend to register its securities under the Securities Act or to conduct a public offering of the securities in the U. S. This presentation does not constitute an offer to the public or an advertisement of securities in the Russian Federation, and is not an offer or an invitation to make offers to purchase securities in the Russian Federation, and must not be passed on to third parties or otherwise made publicly available in the Russian Federation. This communication is only directed (i) at persons who are outside the United Kingdom, (ii) to investment professionals falling within Article 19(5) of the Financial Services and Markets Act of 2000 (Financial Promotion) Order 2005, as amended (the "Order") or (iii) other persons to whom it may be lawfully communicated, falling within Article 49 (a) to (d) of the Order (all such persons together being referred to as "Relevant Persons"). Any person who is not a Relevant Person should not act or rely on this communication or any of its contents. Any investment or investment activity to which this communication relates is available only to relevant persons and will be engaged in only with relevant persons. Other persons should not act or rely on this document or any of its contents. NOT FOR PUBLICATION, RELEASE OR DISTRIBUTION IN THE UNITED STATES, CANADA, AUSTRALIA OR JAPAN. Failure to comply with the restrictions set out in this Disclaimer may constitute a violation of applicable securities laws or may otherwise be actionable. 1

Table of Contents I. Company Overview 3 II. Investment Highlights 9 III. Strategy and directions of development 21 IV. Key Financial Indicators 26 APPENDIX 1. Financial Statements For The Years of 2007 -2010 31 2. Key Financial Parameters Forecast 35 3. Company's Product Portfolio 37 2

Company Overview Section 1

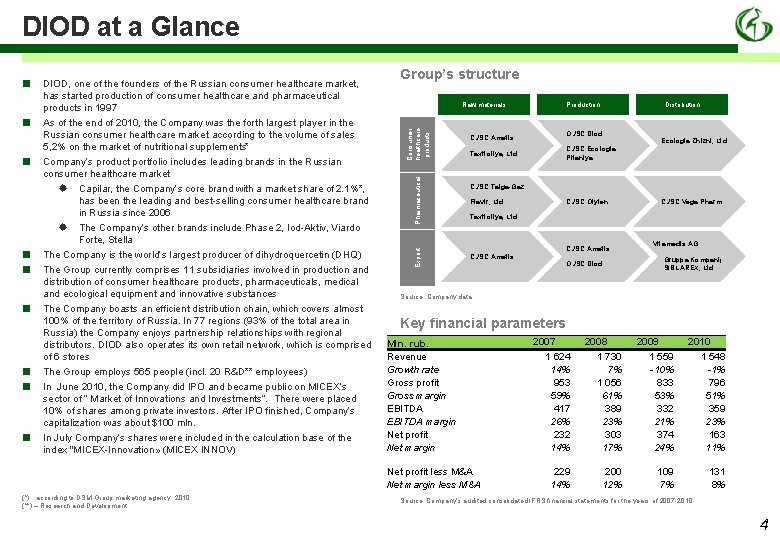
DIOD at a Glance ■ ■ ■ Raw materials Consumer healthcare products ■ Group’s structure Pharmaceutical ■ DIOD, one of the founders of the Russian consumer healthcare market, has started production of consumer healthcare and pharmaceutical products in 1997 As of the end of 2010, the Company was the forth largest player in the Russian consumer healthcare market according to the volume of sales 5, 2% on the market of nutritional supplements* Company’s product portfolio includes leading brands in the Russian consumer healthcare market Capilar, the Company’s core brand with a market share of 2. 1%*, has been the leading and best-selling consumer healthcare brand in Russia since 2006 The Company’s other brands include Phase 2, Iod-Aktiv, Viardo Forte, Stella The Company is the world’s largest producer of dihydroquercetin (DHQ) The Group currently comprises 11 subsidiaries involved in production and distribution of consumer healthcare products, pharmaceuticals, medical and ecological equipment and innovative substances The Company boasts an efficient distribution chain, which covers almost 100% of the territory of Russia. In 77 regions (93% of the total area in Russia) the Company enjoys partnership relationships with regional distributors. DIOD also operates its own retail network, which is comprised of 6 stores The Group employs 565 people (incl. 20 R&D** employees) In June 2010, the Company did IPO and became public on MICEX’s sector of “ Market of Innovations and Investments”. There were placed 10% of shares among private investors. After IPO finished, Company’s capitalization was about $100 mln. In July Company’s shares were included in the calculation base of the index "MICEX-Innovation» (MICEX INNOV) Export ■ Distribution OJSC Diod CJSC Ametis Ecologia Zhizni, Ltd. CJSC Ecologia Pitaniya Taxifoliya, Ltd. CJSC Taiga-Gaz Flavir, Ltd. CJSC Olyfen CJSC Vega Pharm Taxifoliya, Ltd. CJSC Ametis Vitamedis AG Gruppa Kompanij SIBLAREX, Ltd. OJSC Diod Source: Company data Key financial parameters Mln. rub. Revenue Growth rate Gross profit Gross margin EBITDA margin Net profit Net margin Net profit less M&A Net margin less M&A (*) according to DSM Group marketing agency, 2010 (**) – Research and Development Production 2007 2008 2009 2010 1 624 14% 953 59% 417 26% 232 14% 1 730 7% 1 056 61% 389 23% 303 17% 1 559 -10% 833 53% 332 21% 374 24% 1 548 -1% 796 51% 359 23% 163 11% 229 14% 200 12% 109 7% 131 8% Source: Company’s audited consolidated IFRS financial statements for the years of 2007 -2010 4

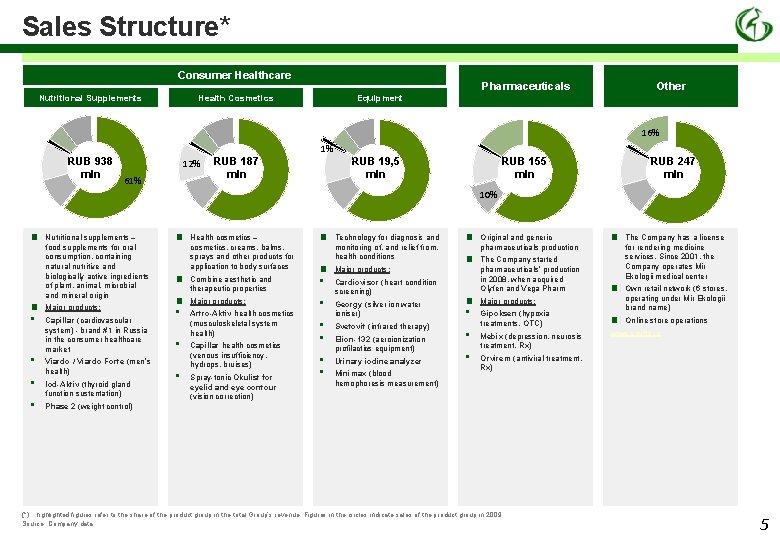
Sales Structure* Consumer Healthcare Nutritional Supplements Pharmaceuticals Equipment Health Cosmetics Other 16% 1% RUB 938 mln 61% 12% RUB 187 mln RUB 19, 5 mln RUB 155 mln RUB 247 mln 10% ■ ■ • • Nutritional supplements – food supplements for oral consumption, containing natural nutritive and biologically active ingredients of plant, animal, microbial and mineral origin Major products: Capillar (cardiovascular system) - brand #1 in Russia in the consumer healthcare market Viardo / Viardo Forte (men’s health) Iod-Aktiv (thyroid gland function sustentation) ■ ■ ■ • • • Health cosmetics – cosmetics, creams, balms, sprays and other products for application to body surfaces Combine aesthetic and therapeutic properties Major products: Artro-Aktiv health cosmetics (musculoskeletal system health) Capillar health cosmetics (venous insufficiency, hydrops, bruises) Spray-tonic Okulist for eyelid and eye contour (vision correction) ■ ■ • • • Technology for diagnosis and monitoring of, and relief from, health conditions Major products: Cardiovisor (heart condition screening) Georgy (silver ion water ioniser) Svetovit (infrared therapy) Elion-132 (aeroionization profilactics equipment) Urinary iodine analyzer Minimax (blood hemophoresis measurement) ■ ■ ■ • • • Original and generic pharmaceuticals production The Company started pharmaceuticals’ production in 2008, when acquired Olyfen and Vega Pharm ■ ■ Major products: The Company has a license for rendering medicine services. Since 2001, the Company operates Mir Ekologii medical center Own retail network (6 stores, operating under Mir Ekologii brand name) Gipoksen (hypoxia treatments, OTC) ■ Mebix (depression, neurosis treatment, Rx) www. ecvita. ru Online store operations Orvirem (antiviral treatment, Rх) Phase 2 (weight control) (*) highlighted figures refer to the share of the product group in the total Group’s revenue. Figures in the circles indicate sales of the product group in 2009 Source: Company data 5

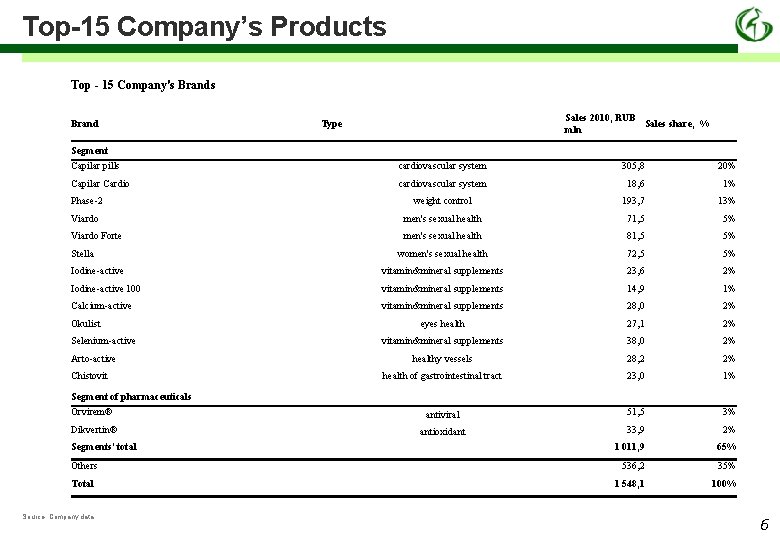
Top-15 Company’s Products Top - 15 Company's Brand Sales 2010, RUB Sales share, % mln Type Segment Capilar pills cardiovascular system 305, 8 20% Capilar Cardio cardiovascular system 18, 6 1% Phase-2 weight control 193, 7 13% Viardo men's sexual health 71, 5 5% Viardo Forte men's sexual health 81, 5 5% women's sexual health 72, 5 5% Iodine-active vitamin&mineral supplements 23, 6 2% Iodine-active 100 vitamin&mineral supplements 14, 9 1% Calcium-active vitamin&mineral supplements 28, 0 2% eyes health 27, 1 2% vitamin&mineral supplements 38, 0 2% healthy vessels 28, 2 2% health of gastrointestinal tract 23, 0 1% Orvirem® antiviral 51, 5 3% Dikvertin® antioxidant 33, 9 2% 1 011, 9 65% 536, 2 35% 1 548, 1 100% Stella Okulist Selenium-active Arto-active Chistovit Segment of pharmaceuticals Segments' total Others Total Source: Company data 6

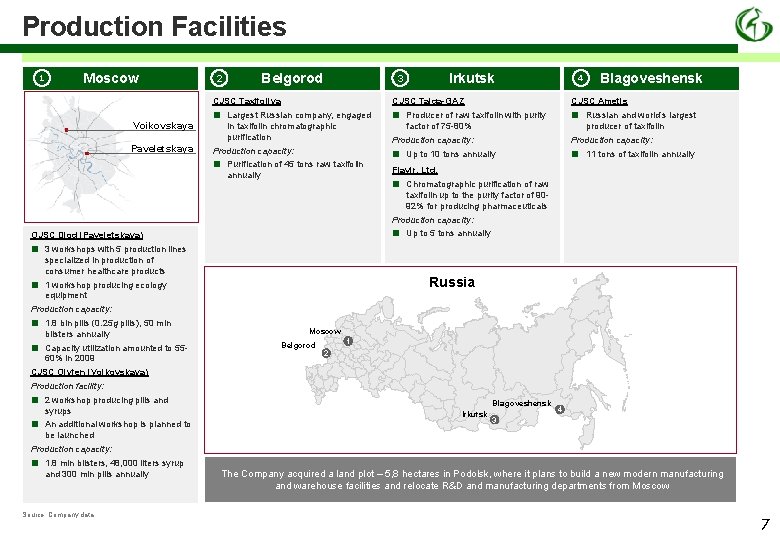
Production Facilities 1 Moscow 2 Belgorod CJSC Taxifoliya Voikovskaya Paveletskaya ■ 4 CJSC Taiga-GAZ Largest Russian company, engaged in taxifolin chromatographic purification Production capacity: ■ Irkutsk 3 Purification of 45 tons raw taxifolin annually ■ CJSC Ametis ■ Producer of raw taxifolin with purity factor of 75 -80% Production capacity: ■ Blagoveshensk Russian and world’s largest producer of taxifolin Production capacity: ■ Up to 10 tons annually 11 tons of taxifolin annually Flavir, Ltd. ■ Chromatographic purification of raw taxifolin up to the purity factor of 9092% for producing pharmaceuticals Production capacity: ■ OJSC Diod (Paveletskaya) ■ ■ 3 workshops with 5 production lines specialized in production of consumer healthcare products Up to 5 tons annually Russia 1 workshop producing ecology equipment Production capacity: ■ ■ 1. 8 bln pills (0. 25 g pills), 50 mln blisters annually Capacity utilization amounted to 5560% in 2009 Moscow Belgorod 1 2 CJSC Olyfen (Voikovskaya) Production facility: ■ ■ 2 workshop producing pills and syrups An additional workshop is planned to be launched Blagoveshensk Irkutsk 4 3 Production capacity: ■ 1. 8 mln blisters, 48, 000 liters syrup and 300 mln pills annually Source: Company data The Company acquired a land plot – 5, 8 hectares in Podolsk, where it plans to build a new modern manufacturing and warehouse facilities and relocate R&D and manufacturing departments from Moscow 7

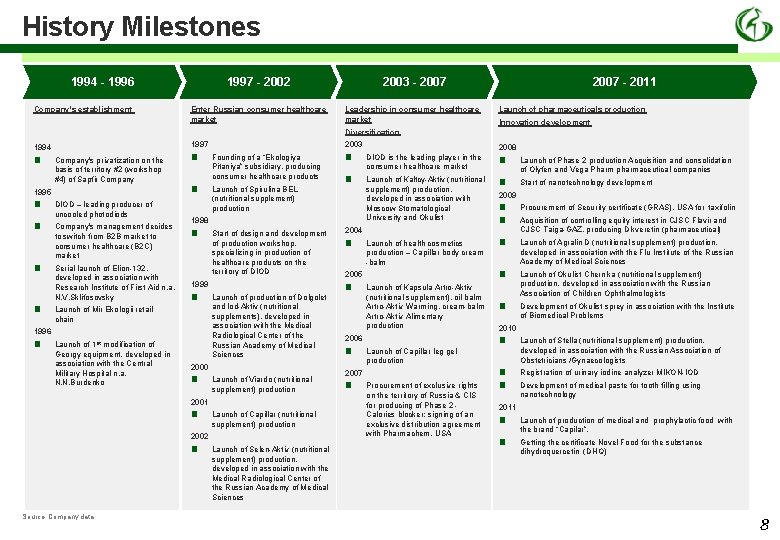
History Milestones 1994 - 1996 Company's establishment 1997 - 2002 Enter Russian consumer healthcare market 2003 - 2007 Leadership in consumer healthcare market 2007 - 2011 Launch of pharmaceuticals production Innovation development Diversification 1997 1994 ■ Company's privatization on the basis of territory #2 (workshop #4) of Sapfir Company 1995 ■ ■ DIOD – leading producer of uncooled photodiods Company's management decides to switch from B 2 B market to consumer healthcare (B 2 C) market Serial launch of Elion-132, developed in association with Research Institute of First Aid n. a. N. V. Sklifosovsky ■ ■ ■ Start of design and development of production workshop, specializing in production of healthcare products on the territory of DIOD 1999 ■ Launch of Mir Ekologii retail chain Launch of 1 st modification of Georgy equipment, developed in association with the Central Military Hospital n. a. N. N. Burdenko Launch of Spirulina BEL (nutritional supplement) production Launch of production of Dolgolet and Iod-Aktiv (nutritional supplements), developed in association with the Medical Radiological Center of the Russian Academy of Medical Sciences 2000 ■ Launch of Viardo (nutritional supplement) production 2001 ■ Launch of Capillar (nutritional supplement) production 2002 ■ Source: Company data ■ ■ 1998 1996 ■ 2003 Founding of a “Ekologiya Pitaniya” subsidiary, producing consumer healthcare products Launch of Selen-Aktiv (nutritional supplement) production, developed in association with the Medical Radiological Center of the Russian Academy of Medical Sciences 2008 DIOD is the leading player in the consumer healthcare market ■ Launch of Kaltcy-Aktiv (nutritional supplement) production, developed in association with Moscow Stomatological University and Okulist ■ 2004 ■ Launch of health cosmetics production – Capillar body cream -balm 2005 ■ Launch of Kapsula Artro-Aktiv (nutritional supplement), oil balm Artro-Aktiv Warming, cream-balm Artro-Aktiv Alimentary production ■ Launch of Capillar leg gel production 2007 ■ Procurement of exclusive rights on the territory of Russia & CIS for producing of Phase 2 Calories blocker; signing of an exclusive distribution agreement with Pharmachem, USA Start of nanotechnology development 2009 ■ ■ ■ Procurement of Security certificate (GRAS), USA for taxifolin Acquisition of controlling equity interest in CJSC Flavir and CJSC Taiga-GAZ, producing Dikveretin (pharmaceutical) Launch of Agralin D (nutritional supplement) production, developed in association with the Flu Institute of the Russian Academy of Medical Sciences Launch of Okulist Chernika (nutritional supplement) production, developed in association with the Russian Association of Children Ophthalmologists Development of Okulist sprey in association with the Institute of Biomedical Problems 2010 ■ 2006 Launch of Phase 2 production Acquisition and consolidation of Olyfen and Vega Pharm pharmaceutical companies ■ ■ Launch of Stella (nutritional supplement) production, developed in association with the Russian Association of Obstetricians /Gynaecologists Registration of urinary iodine analyzer MIKON-IOD Development of medical paste for tooth filling using nanotechnology 2011 ■ ■ Launch of production of medical and prophylactic food with the brand “Capilar”. Getting the certificate Novel Food for the substance dihydroquercetin (DHQ) 8

Investment Highlights Section 2

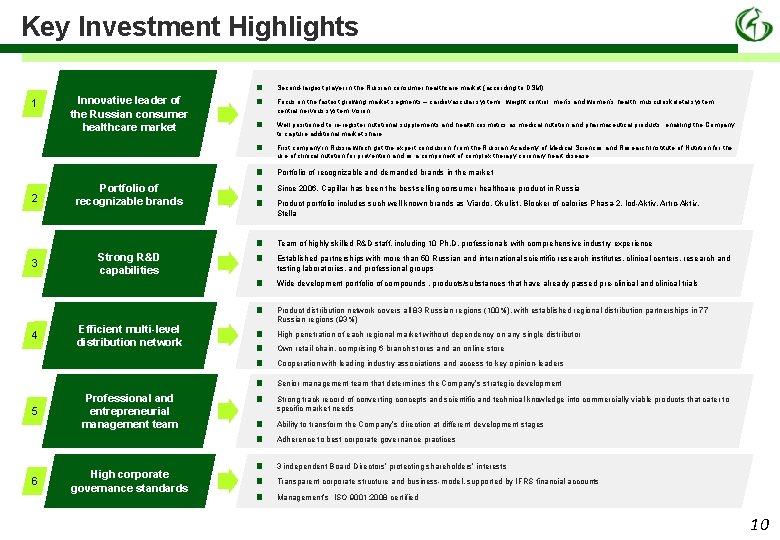
Key Investment Highlights 1 Innovative leader of the Russian consumer healthcare market ■ ■ 2 3 Portfolio of recognizable brands Strong R&D capabilities ■ ■ ■ ■ 4 5 6 Efficient multi-level distribution network Professional and entrepreneurial management team High corporate governance standards ■ ■ ■ ■ ■ Second-largest player in the Russian consumer healthcare market (according to DSM) Focus on the fastest growing market segments – cardiovascular systems, weight control, men’s and women’s health, musculoskeletal system, central nervous system, vision Well positioned to re-register nutritional supplements and health cosmetics as medical nutrition and pharmaceutical products, enabling the Company to capture additional market share First company in Russia which got the expert conclusion from the Russian Academy of Medical Sciences and Research Institute of Nutrition for the use of clinical nutrition for prevention and as a component of complex therapy coronary heart disease Portfolio of recognizable and demanded brands in the market Since 2006, Capillar has been the best-selling consumer healthcare product in Russia Product portfolio includes such well-known brands as Viardo, Okulist, Blocker of calories Phasa-2, Iod-Aktiv, Artro-Aktiv, Stella Team of highly skilled R&D staff, including 10 Ph. D. professionals with comprehensive industry experience Established partnerships with more than 50 Russian and international scientific research institutes, clinical centers, research and testing laboratories, and professional groups Wide development portfolio of compounds , products/substances that have already passed pre-clinical and clinical trials Product distribution network covers all 83 Russian regions (100%), with established regional distribution partnerships in 77 Russian regions (93%) High penetration of each regional market without dependency on any single distributor Own retail chain, comprising 6 branch stores and an online store Cooperation with leading industry associations and access to key opinion-leaders Senior management team that determines the Company’s strategic development Strong track record of converting concepts and scientific and technical knowledge into commercially viable products that cater to specific market needs Ability to transform the Company’s direction at different development stages Adherence to best corporate governance practices 3 independent Board Directors’ protecting shareholders’ interests Transparent corporate structure and business-model, supported by IFRS financial accounts Management’s ISO 9001: 2008 certified 10

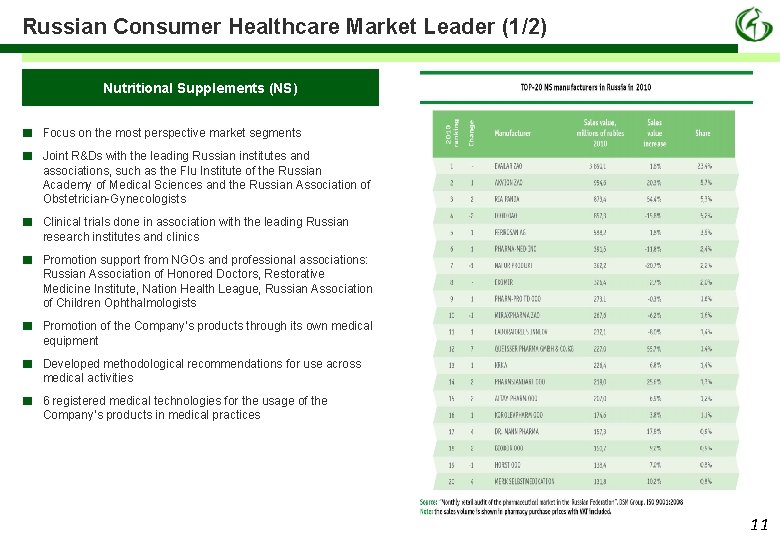
Russian Consumer Healthcare Market Leader (1/2) Nutritional Supplements (NS) ■ ■ Focus on the most perspective market segments ■ Clinical trials done in association with the leading Russian research institutes and clinics ■ Promotion support from NGOs and professional associations: Russian Association of Honored Doctors, Restorative Medicine Institute, Nation Health League, Russian Association of Children Ophthalmologists ■ Promotion of the Company’s products through its own medical equipment ■ Developed methodological recommendations for use across medical activities ■ 6 registered medical technologies for the usage of the Company’s products in medical practices Joint R&Ds with the leading Russian institutes and associations, such as the Flu Institute of the Russian Academy of Medical Sciences and the Russian Association of Obstetrician-Gynecologists 11

Russian Consumer Healthcare Market Leader (2/2) Health Cosmetics ■ ■ ■ As of 2010, the Company’s market share in the health cosmetics market amounted to 3. 4% Ability to leverage off some of the best selling brands to produce a number of successful brand families The Company registered 3 medical technologies for the usage of health cosmetics products in medical practices Equipment ■ ■ The Company produces diagnostic and therapeutic medical equipment ■ The Company registered 2 medical technologies for the usage of its products in medical practices (on the basis of Georgy water ionizer and Svetovit infrared therapy equipment) Medical equipment allows the Company to effectively promote other Company’s products as well as to demonstrate effectiveness of the Company’s consumer healthcare products Source: DSM Group data 12

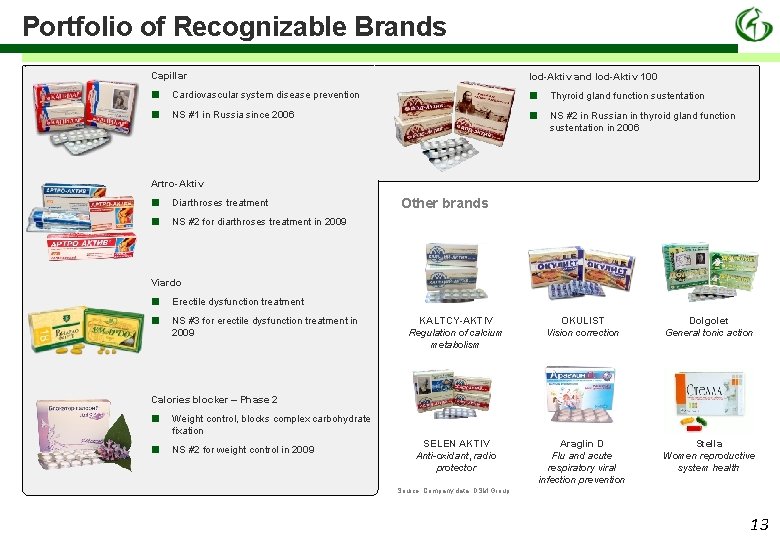
Portfolio of Recognizable Brands Capillar Iod-Aktiv and Iod-Aktiv 100 ■ ■ Cardiovascular system disease prevention NS #1 in Russia since 2006 Thyroid gland function sustentation NS #2 in Russian in thyroid gland function sustentation in 2006 Artro-Aktiv ■ ■ Diarthroses treatment Other brands NS #2 for diarthroses treatment in 2009 Viardo ■ ■ Erectile dysfunction treatment NS #3 for erectile dysfunction treatment in 2009 KALTCY-AKTIV Regulation of calcium metabolism OKULIST Vision correction Dolgolet General tonic action SELEN AKTIV Anti-oxidant, radio protector Araglin D Flu and acute respiratory viral infection prevention Stella Women reproductive system health Calories blocker – Phase 2 ■ Weight control, blocks complex carbohydrate fixation ■ NS #2 for weight control in 2009 Source: Company data, DSM Group 13

Clinical nutrition (1/2) ■ ■ JSC DIOD opens a new manufacturing segment – clinical nutrition. ■ JSC DIOD has got Certificate of State registration of clinical (dietary) nutrition “Cardio capilar”, “Capilar 120/80”. ■ In the nearest future JSC DIOD is planning to produce clinical nutrition for use together with cure as a complex therapy of treatment the following diseases: arthrosis, vision diseases, insufficiency of iodine, diseases of female reproductive system. This is relatively new segment of products for health for Russia. Production of clinical and functional nutrition is the main brand of Healthcare industry. Certificates of state registration of clinical (dietary) nutrition ‘’Cardio capilar with coenzyme Q 10”, “Capilar 120/80” is for: ■ strengthening of vascular walls and improvement of functional state of cardiovascular system; ■ ■ normalization of arterial pressure and heart rate; reduction of risk of development of ischemic heart disease, stroke, hypertension, atherosclerosis. “Cardio capilar” is for: ■ reduction of risk of development of ischemic heart disease and its complications (heart attack and stroke), cardiopulmonary insufficiency; ■ Reduction of inclination to trombus’ formation, normalization of microcirculation of blood, improvement of functional state of cardiovascular system; ■ For realization to people who suffer from high arterial pressure and hypertension For realization to people as specialized foodstuff for dietary (clinical and prophylactic) nutrition for prevention and complex therapy of ischemia Consumer package Increase of physical endurance of heart muscle and tolerance to physical activity, reduction of period of rehabilitation after acute old myocardial infarction. 14

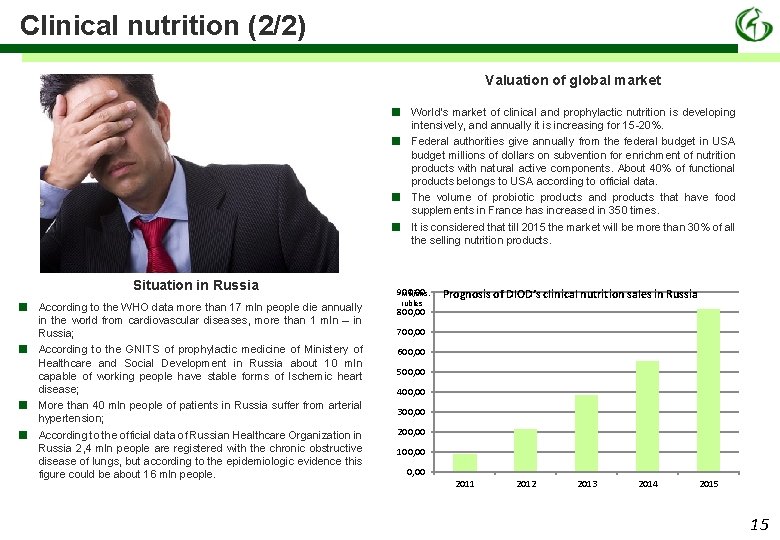
Clinical nutrition (2/2) Valuation of global market ■ ■ Situation in Russia ■ ■ According to the WHO data more than 17 mln people die annually in the world from cardiovascular diseases, more than 1 mln – in Russia; According to the GNITS of prophylactic medicine of Ministery of Healthcare and Social Development in Russia about 10 mln capable of working people have stable forms of Ischemic heart disease; More than 40 mln people of patients in Russia suffer from arterial hypertension; According to the official data of Russian Healthcare Organization in Russia 2, 4 mln people are registered with the chronic obstructive disease of lungs, but according to the epidemiologic evidence this figure could be about 16 mln people. World’s market of clinical and prophylactic nutrition is developing intensively, and annually it is increasing for 15 -20%. Federal authorities give annually from the federal budget in USA budget millions of dollars on subvention for enrichment of nutrition products with natural active components. About 40% of functional products belongs to USA according to official data. The volume of probiotic products and products that have food supplements in France has increased in 350 times. It is considered that till 2015 the market will be more than 30% of all the selling nutrition products. 900, 00 Millions, rubles Prognosis of DIOD’s clinical nutrition sales in Russia 800, 00 700, 00 600, 00 500, 00 400, 00 300, 00 200, 00 100, 00 2011 2012 2013 2014 2015 15

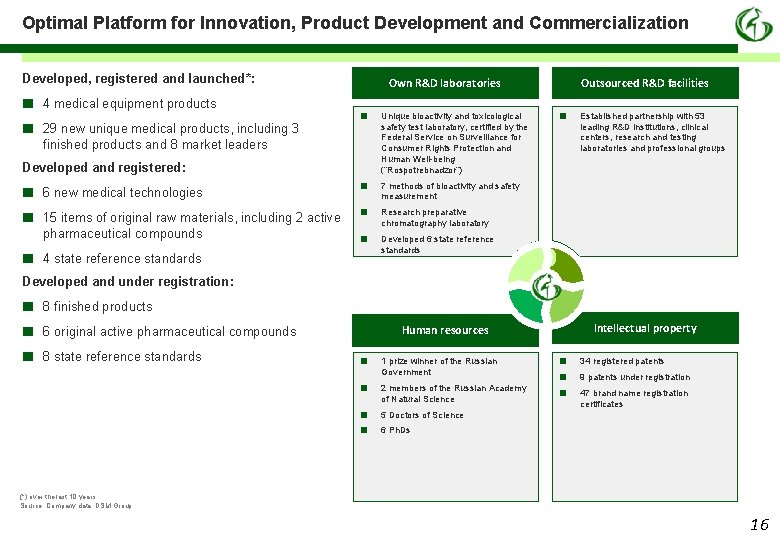
Optimal Platform for Innovation, Product Development and Commercialization Developed, registered and launched*: ■ ■ 4 medical equipment products 29 new unique medical products, including 3 finished products and 8 market leaders Own R&D laboratories ■ Developed and registered: ■ ■ ■ 6 new medical technologies 15 items of original raw materials, including 2 active pharmaceutical compounds ■ ■ ■ 4 state reference standards Unique bioactivity and toxicological safety test laboratory, certified by the Federal Service on Surveillance for Consumer Rights Protection and Human Well-being (“Rospotrebnadzor”) Outsourced R&D facilities ■ Established partnership with 53 leading R&D institutions, clinical centers, research and testing laboratories and professional groups 7 methods of bioactivity and safety measurement Research preparative chromatography laboratory Developed 6 state reference standards Developed and under registration: ■ ■ ■ 8 finished products 8 state reference standards Intellectual property Human resources 6 original active pharmaceutical compounds ■ ■ 1 prize winner of the Russian Government 2 members of the Russian Academy of Natural Science ■ ■ ■ 34 registered patents 9 patents under registration 47 brand name registration certificates 5 Doctors of Science 6 Ph. Ds (*) over the last 10 years Source: Company data, DSM Group 16

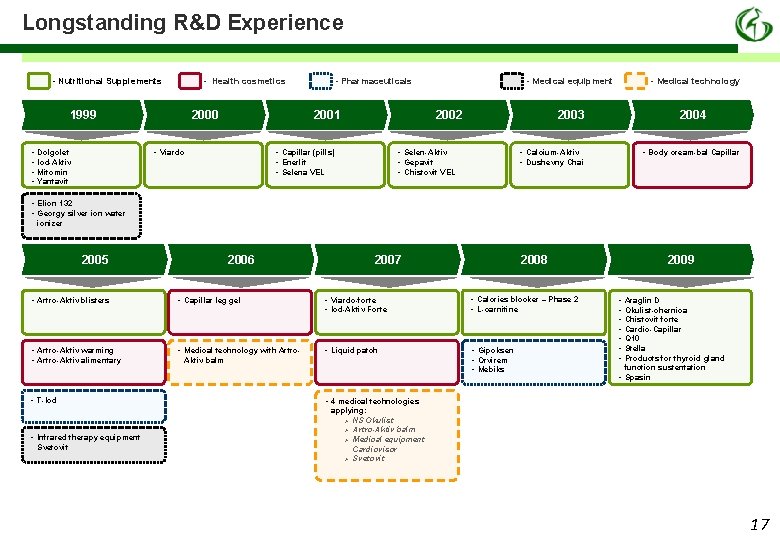
Longstanding R&D Experience - Nutritional Supplements - Health cosmetics 1999 • • 2000 2001 • Viardo Dolgolet Iod-Aktiv Mitomin Yantavit - Pharmaceuticals - Medical equipment 2002 • Capillar (pills) • Enerlit • Selena VEL - Medical technology 2003 • Selen-Aktiv • Gepavit • Chistovit VEL 2004 • Calcium-Aktiv • Dushevny Chai • Body cream-bal Capillar • Elion 132 • Georgy silver ion water ionizer 2005 2006 2007 2008 • Artro-Aktiv blisters • Capillar leg gel • Viardo-forte • Iod-Aktiv Forte • Calories blocker – Phase 2 • L-carnitine • Artro-Aktiv warming • Artro-Aktiv alimentary • Medical technology with Artro- • Liquid patch • Gipoksen • Orvirem • Mebiks • T-Iod • Infrared therapy equipment Svetovit Aktiv balm 2009 • • Araglin D Okulist-chernica Chistovit forte Cardio-Capillar Q 10 Stella Products for thyroid gland function sustentation • Spasin • 4 medical technologies applying: Ø NS Okulist Ø Artro-Aktiv balm Ø Medical equipment Cardiovisor Ø Svetovit 17

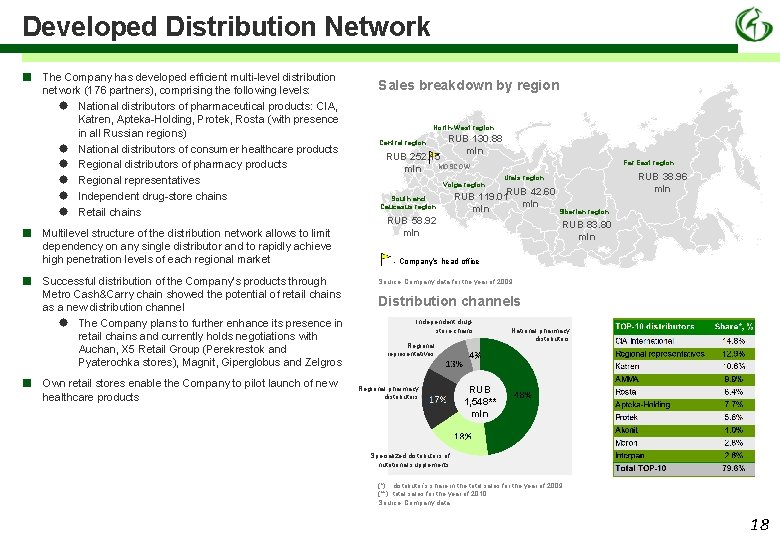
Developed Distribution Network ■ ■ The Company has developed efficient multi-level distribution network (176 partners), comprising the following levels: National distributors of pharmaceutical products: CIA, Katren, Apteka-Holding, Protek, Rosta (with presence in all Russian regions) National distributors of consumer healthcare products Regional distributors of pharmacy products Regional representatives Independent drug-store chains Retail chains Multilevel structure of the distribution network allows to limit dependency on any single distributor and to rapidly achieve high penetration levels of each regional market Successful distribution of the Company’s products through Metro Cash&Carry chain showed the potential of retail chains as a new distribution channel The Company plans to further enhance its presence in retail chains and currently holds negotiations with Auchan, X 5 Retail Group (Perekrestok and Pyaterochka stores), Magnit, Giperglobus and Zelgros Own retail stores enable the Company to pilot launch of new healthcare products Sales breakdown by region North-West region Central region RUB 130. 88 mln RUB 252. 15 MOSCOW mln Volga region South and Caucasus region Far East region RUB 38. 96 mln Urals region RUB 42. 60 RUB 119. 01 mln RUB 58. 92 mln Siberian region RUB 83. 80 mln - Company’s head office Source: Company data for the year of 2009 Distribution channels Independent drugstore chains Regional representatives Regional pharmacy distributors National pharmacy distributors RUB 1, 548** mln Specialized distributors of nutritional supplements (*) distributor’s share in the total sales for the year of 2009 (**) total sales for the year of 2010 Source: Company data 18

Experienced Management Team The team of professionals with long-term experience in the market Vladimir Tikhonov CEO One of the Company’s founders, who manages it since its origin in 1994. Graduated from Moscow State Technical University n. a. Bauman in 1992. Member of the Russian Academy of Natural Science Vladimir Kozlov First Deputy CEO One of the Company’s founders, Chairman of the Board of Directors. Graduated from Moscow Radiotechnics, Electronics and Automation Institute in 1991 Eduard Chernyshov Foreign Economic Relations Director Started his career in OJSC DIOD in the beginning of 1995. Graduated from All-Union Extramural Polytechnics Institute in 1989 Vadim Silantiev Sales and Marketing Director Occupies this position since the end of 2000. Graduated from All-Russian Extramural Financial-Economics Institute in 2002 Lubov Dergacheva Head of R&D Department Occupies this position from September 2004. Graduated from Moscow State Medical University in 1984. Highest Quality Endocrinologist, specialist in restorative medicine, holds GSP certificate 19

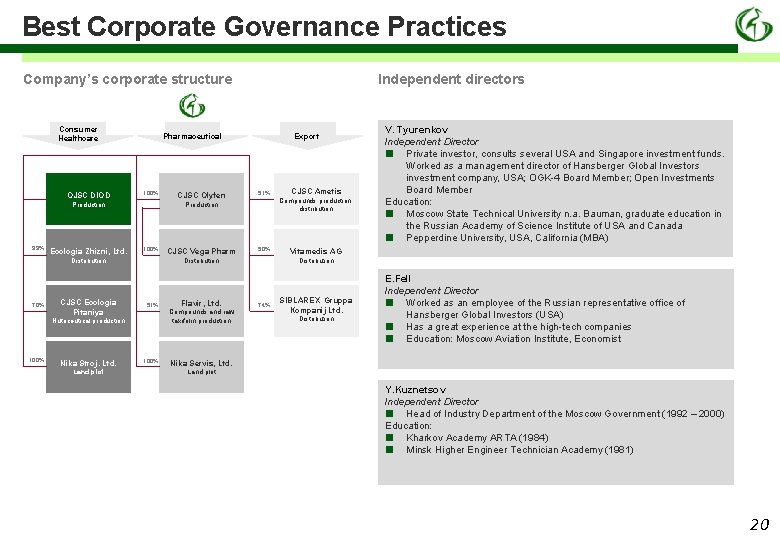
Best Corporate Governance Practices Company’s corporate structure Consumer Healthcare OJSC DIOD Pharmaceutical 100% Production 99% Ecologia Zhizni, Ltd. CJSC Ecologia Pitaniya 100% Nika Stroj. Ltd. Land plot 51% CJSC Vega Pharm 51% 100% Flavir, Ltd. Compounds and raw taxifolin production CJSC Ametis Compounds production, distribution 50% Distribution Nutriceutical production 100% CJSC Olyfen Export Production Distribution 70% Independent directors V. Tyurenkov Independent Director ■ Private investor, consults several USA and Singapore investment funds. Worked as a management director of Hansberger Global Investors investment company, USA; OGK-4 Board Member; Open Investments Board Member Education: ■ Moscow State Technical University n. a. Bauman, graduate education in the Russian Academy of Science Institute of USA and Canada ■ Pepperdine University, USA, California (MBA) Vitamedis AG Distribution 74% SIBLAREX Gruppa Kompanij Ltd. Distribution E. Fell Independent Director ■ Worked as an employee of the Russian representative office of Hansberger Global Investors (USA) ■ Has a great experience at the high-tech companies ■ Education: Moscow Aviation Institute, Economist Nika Servis, Ltd. Land plot Y. Kuznetsov Independent Director ■ Head of Industry Department of the Moscow Government (1992 – 2000) Education: ■ Kharkov Academy АRTA (1984) ■ Minsk Higher Engineer Technician Academy (1981) 20

Strategy and directions of development Section 3

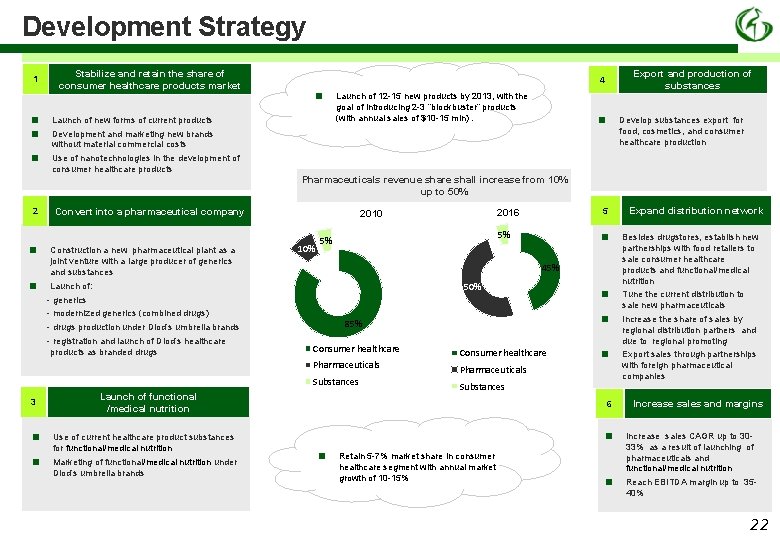
Development Strategy 1 ■ ■ ■ 2 ■ ■ Stabilize and retain the share of consumer healthcare products market ■ Launch of new forms of current products Launch of 12 -15 new products by 2013, with the goal of introducing 2 -3 “blockbuster” products (with annual sales of $10 -15 mln). ■ Use of nanotechnologies in the development of consumer healthcare products Pharmaceuticals revenue share shall increase from 10% up to 50% Convert into a pharmaceutical company Construction a new pharmaceutical plant as a joint venture with a large producer of generics and substances 2010 10% 5% 2016 5 5% ■ 45% Launch of: 50% - modernized generics (combined drugs) - registration and launch of Diod’s healthcare products as branded drugs Consumer healthcare Pharmaceuticals Substances ■ Launch of functional /medical nutrition Use of current healthcare product substances for functional/medical nutrition Marketing of functional/medical nutrition under Diod’s umbrella brands ■ ■ 85% - drugs production under Diod’s umbrella brands ■ Develop substances export for food, cosmetics, and consumer healthcare production Development and marketing new brands without material commercial costs - generics 3 Export and production of substances 4 Consumer healthcare ■ Pharmaceuticals Substances 6 ■ ■ Retain 5 -7% market share in consumer healthcare segment with annual market growth of 10 -15% ■ Expand distribution network Besides drugstores, establish new partnerships with food retailers to sale consumer healthcare products and functional/medical nutrition Tune the current distribution to sale new pharmaceuticals Increase the share of sales by regional distribution partners and due to regional promoting Export sales through partnerships with foreign pharmaceutical companies Increase sales and margins Increase sales CAGR up to 3033% as a result of launching of pharmaceuticals and functional/medical nutrition Reach EBITDA margin up to 3540% 22

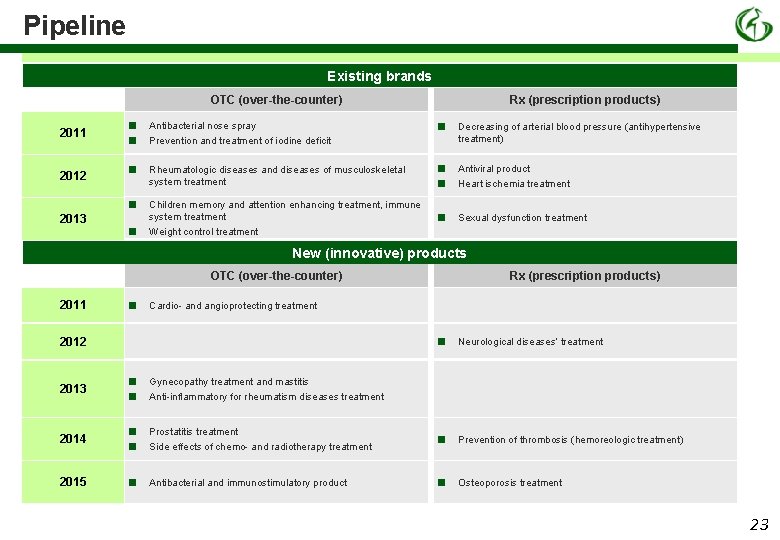
Pipeline Existing brands OTC (over-the-counter) 2011 2012 2013 ■ ■ Antibacterial nose spray Prevention and treatment of iodine deficit ■ ■ ■ Rx (prescription products) ■ Decreasing of arterial blood pressure (antihypertensive treatment) Rheumatologic diseases and diseases of musculoskeletal system treatment ■ ■ Antiviral product Heart ischemia treatment Children memory and attention enhancing treatment, immune system treatment ■ Sexual dysfunction treatment Weight control treatment New (innovative) products OTC (over-the-counter) 2011 ■ Rx (prescription products) Cardio- and angioprotecting treatment 2012 ■ Neurological diseases’ treatment 2013 ■ ■ Gynecopathy treatment and mastitis Anti-inflammatory for rheumatism diseases treatment 2014 ■ ■ Prostatitis treatment Side effects of chemo- and radiotherapy treatment ■ Prevention of thrombosis (hemoreologic treatment) 2015 ■ Antibacterial and immunostimulatory product ■ Osteoporosis treatment 23

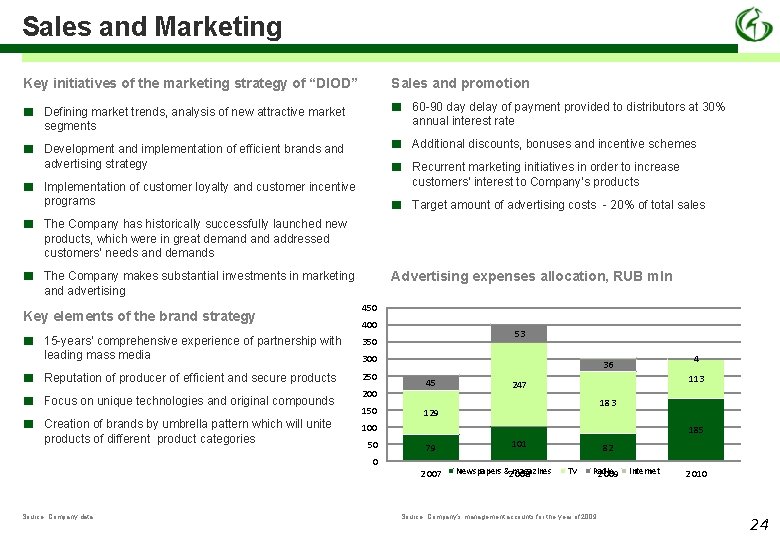
Sales and Marketing Key initiatives of the marketing strategy of “DIOD” Sales and promotion ■ Defining market trends, analysis of new attractive market segments ■ 60 -90 day delay of payment provided to distributors at 30% annual interest rate ■ Development and implementation of efficient brands and advertising strategy ■ ■ Additional discounts, bonuses and incentive schemes Implementation of customer loyalty and customer incentive programs ■ Target amount of advertising costs - 20% of total sales ■ ■ The Company has historically successfully launched new products, which were in great demand addressed customers’ needs and demands ■ The Company makes substantial investments in marketing and advertising Key elements of the brand strategy ■ ■ Advertising expenses allocation, RUB mln 450 400 15 -years’ comprehensive experience of partnership with leading mass media 350 Reputation of producer of efficient and secure products 250 Focus on unique technologies and original compounds Creation of brands by umbrella pattern which will unite products of different product categories Recurrent marketing initiatives in order to increase customers’ interest to Company’s products 53 300 200 150 45 113 247 183 129 100 50 185 79 0 2 007 Source: Company data 4 36 101 Newspapers &2 magazines 008 82 TV Radio 2 009 Source: Company’s management accounts for the year of 2009 Internet 2 010 24

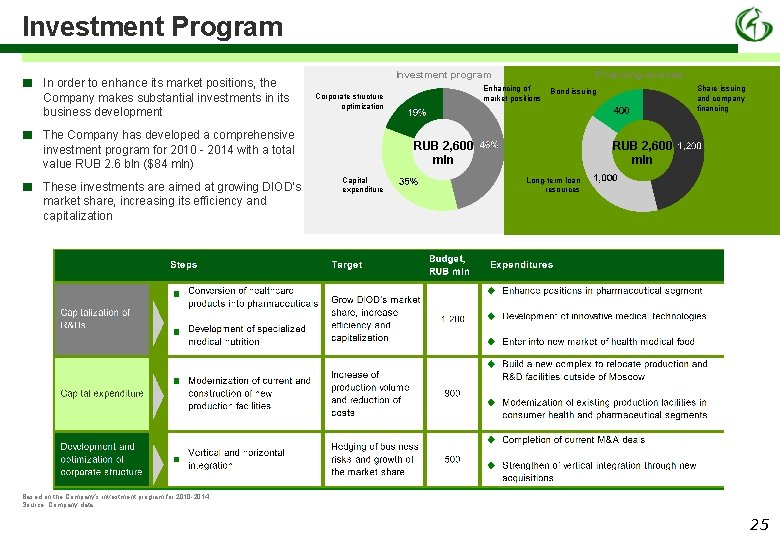
Investment Program ■ In order to enhance its market positions, the Company makes substantial investments in its business development ■ The Company has developed a comprehensive investment program for 2010 - 2014 with a total value RUB 2. 6 bln ($84 mln) ■ These investments are aimed at growing DIOD’s market share, increasing its efficiency and capitalization Investment program Financing sources Enhancing of market positions Corporate structure optimization RUB 2, 600 mln Capital expenditure Share issuing and company financing Bond issuing RUB 2, 600 mln Long-term loan resources Based on the Company’s investment program for 2010 -2014. Source: Company data. 25

Key Financial Indicators Section 4

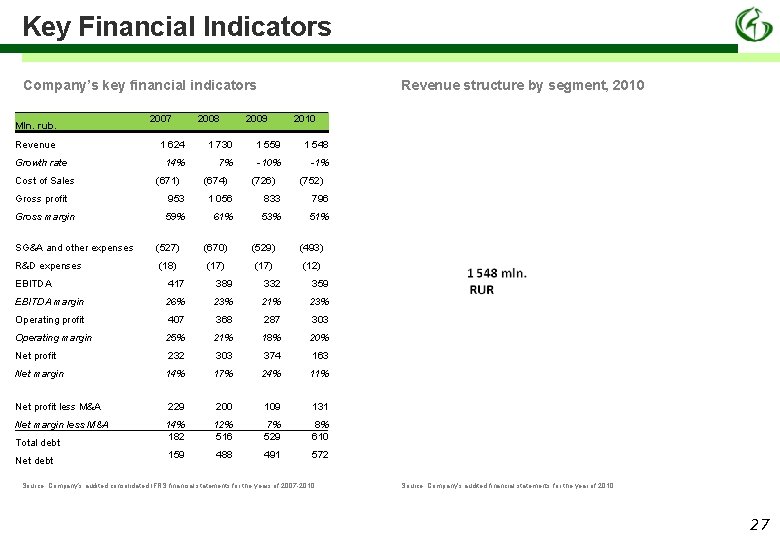
Key Financial Indicators Company’s key financial indicators Mln. rub. Revenue Growth rate Cost of Sales Gross profit Gross margin 2007 2008 Revenue structure by segment, 2010 2009 2010 1 624 1 730 1 559 1 548 14% 7% -10% -1% (671) (674) (726) (752) 953 1 056 833 796 59% 61% 53% 51% SG&A and other expenses (527) (670) (529) (493) R&D expenses (18) (17) (12) EBITDA 417 389 332 359 EBITDA margin 26% 23% 21% 23% Operating profit 407 368 287 303 25% 21% 18% 20% 232 303 374 163 14% 17% 24% 11% 229 200 109 131 14% 182 12% 516 7% 529 8% 610 159 488 491 572 Operating margin Net profit Net margin Net profit less M&A Net margin less M&A Total debt Net debt Source: Company’s audited consolidated IFRS financial statements for the years of 2007 -2010 Source: Company’s audited financial statements for the year of 2010 27

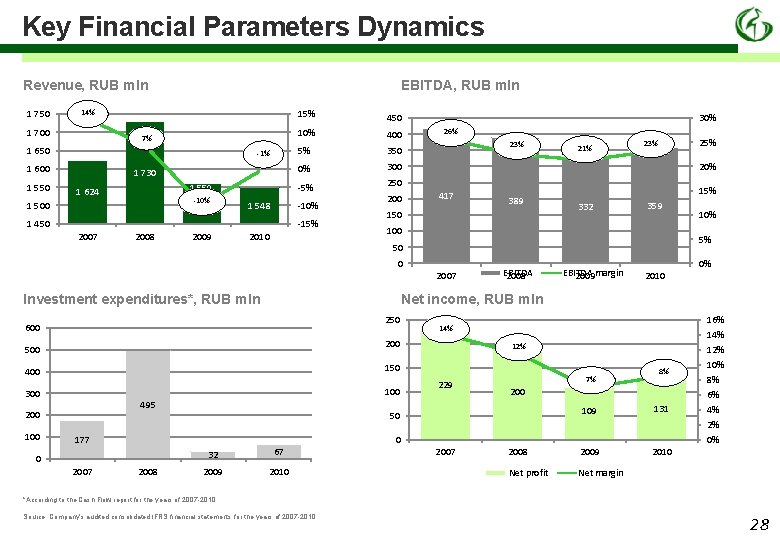
Key Financial Parameters Dynamics Revenue, RUB mln 1 750 EBITDA, RUB mln 14% 1 700 15% 7% 1 650 -1% 1 600 1 550 1 730 1 559 1 624 -10% 1 500 400 5% 350 0% 300 -5% 250 -10% 1 548 1 450 -15% 2007 2008 2009 450 10% 2010 200 30% 26% 23% 417 10% 5% EBITDA 2008 EBITDA 2009 margin 0% 2010 14% 200 500 400 150 300 100 495 2008 359 Net income, RUB mln 250 2007 332 50 600 12% 229 7% 32 67 2009 2010 8% 200 109 131 2008 2009 2010 Net profit Net margin 50 177 15% 100 Investment expenditures*, RUB mln 0 389 150 2007 100 25% 23% 20% 0 200 21% 0 2007 16% 14% 12% 10% 8% 6% 4% 2% 0% *According to the Cash Flow report for the years of 2007 -2010 Source: Company’s audited consolidated IFRS financial statements for the years of 2007 -2010 28

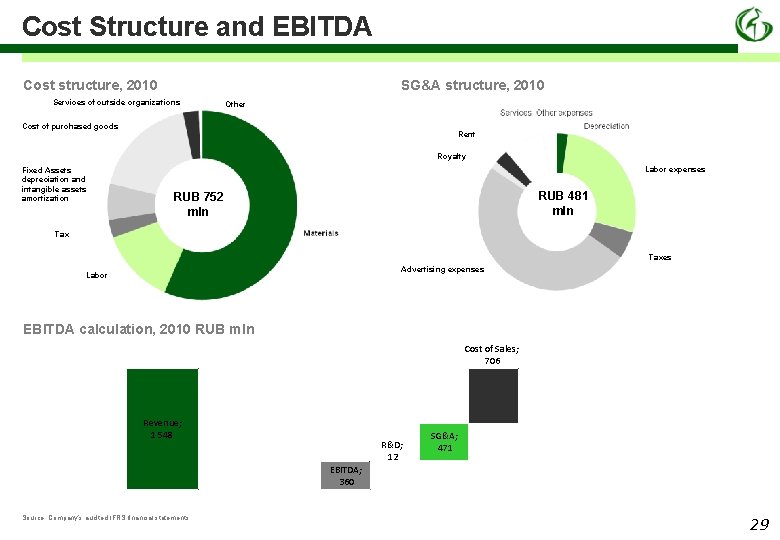
Cost Structure and EBITDA Cost structure, 2010 SG&A structure, 2010 Services of outside organizations Other Cost of purchased goods Rent Royalty Fixed Assets depreciation and intangible assets amortization Labor expenses RUB 481 mln RUB 752 mln Taxes Advertising expenses Labor EBITDA calculation, 2010 RUB mln Cost of Sales; 706 Revenue; 1 548 R&D; 12 SG&A; 471 EBITDA; 360 Source: Company’s audited IFRS financial statements 29

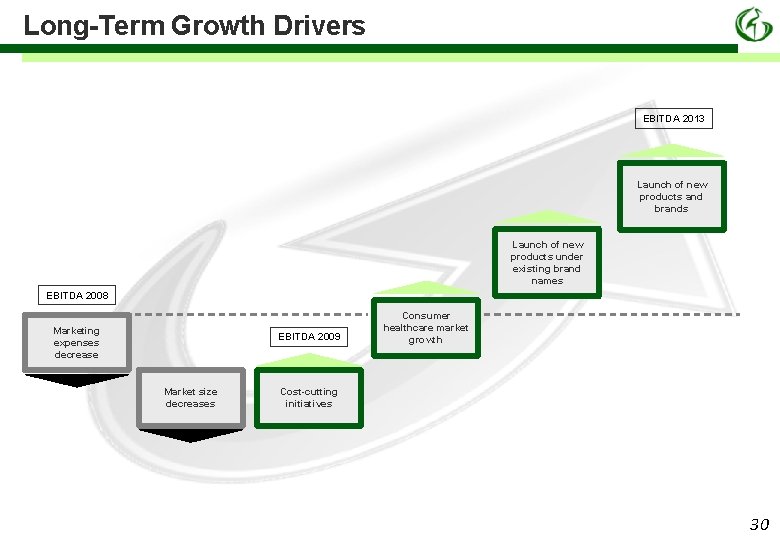
Long-Term Growth Drivers EBITDA 2013 Launch of new products and brands Launch of new products under existing brand names EBITDA 2008 Marketing expenses decrease EBITDA 2009 Market size decreases Consumer healthcare market growth Cost-cutting initiatives 30

Financial Statements for the Years of 2007 -2010 Appendix 1

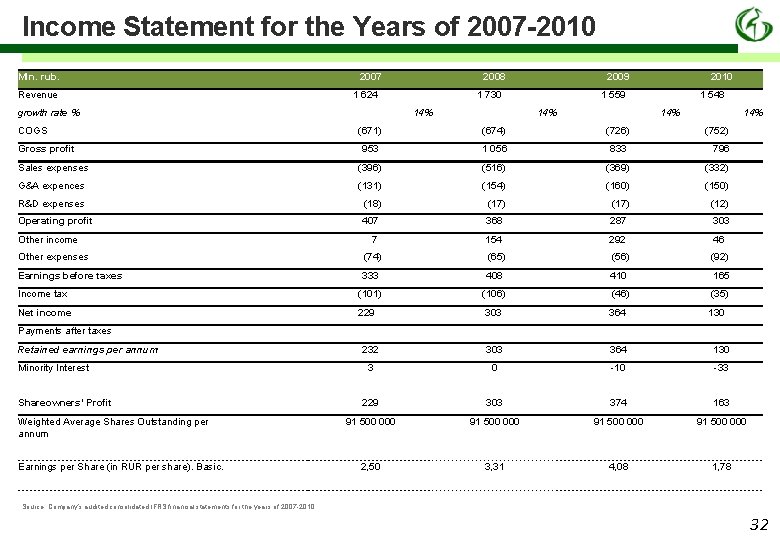
Income Statement for the Years of 2007 -2010 Mln. rub. Revenue 2007 1 624 growth rate % COGS 2008 2009 1 730 14% 2010 1 559 14% 1 548 14% (671) (674) (726) (752) 953 1 056 833 796 Sales expenses (396) (516) (369) (332) G&A expences (131) (154) (160) (150) R&D expenses (18) (17) (12) Operating profit 407 368 287 303 7 154 292 46 Gross profit Other income Other expenses (74) (65) (56) (92) Earnings before taxes 333 408 410 165 Income tax (101) (106) (46) (35) Net income 229 303 364 130 232 303 364 130 3 0 -10 -33 229 303 374 163 91 500 000 2, 50 3, 31 4, 08 1, 78 Payments after taxes Retained earnings per annum Minority Interest Shareowners’ Profit Weighted Average Shares Outstanding per annum Earnings per Share (in RUR per share). Basic. Source: Company’s audited consolidated IFRS financial statements for the years of 2007 -2010 32

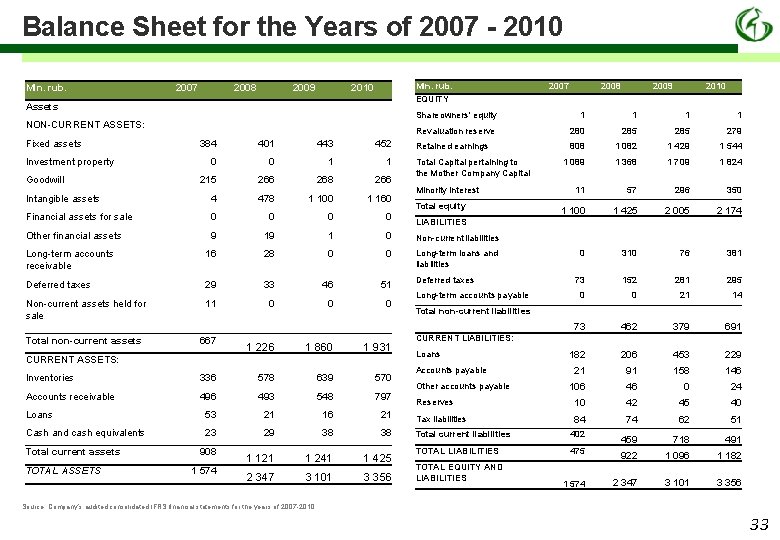
Balance Sheet for the Years of 2007 - 2010 Mln. rub. 2007 2008 2009 Mln. rub. EQUITY 2010 Assets NON-CURRENT ASSETS: Fixed assets 1 1 Revaluation reserve 280 285 279 Retained earnings 808 1 082 1 429 1 544 1 089 1 368 1 709 1 824 11 57 296 350 1 100 1 425 2 005 2 174 0 310 76 381 73 152 281 295 0 0 21 14 73 462 379 691 182 206 453 229 21 91 158 146 106 46 0 24 452 0 0 1 1 215 266 268 266 Intangible assets 4 478 1 100 1 160 Financial assets for sale 0 0 Other financial assets 9 19 1 0 Non-current liabilities Long-term accounts receivable 16 28 0 0 Long-term loans and liabilities Deferred taxes 29 33 46 51 Non-current assets held for sale 11 0 0 0 Total non-current assets 667 1 226 1 860 1 931 2010 1 443 CURRENT ASSETS: 2009 1 401 Goodwill 2008 Shareowners' equity 384 Investment property 2007 Total Capital pertaining to the Mother Company Capital Minority interest Total equity LIABILITIES Deferred taxes Long-term accounts payable Total non-current liabilities CURRENT LIABILITIES: Loans Accounts payable Inventories 336 578 639 570 Accounts receivable 496 493 548 797 Reserves 10 42 45 40 Loans 53 21 16 21 Tax liabilities 84 74 62 51 Cash and cash equivalents 23 29 38 38 Total current liabilities 402 459 718 491 TOTAL LIABILITIES 475 922 1 096 1 182 1 574 2 347 3 101 3 356 Total current assets TOTAL ASSETS 908 1 574 1 121 1 241 1 425 2 347 3 101 3 356 Other accounts payable TOTAL EQUITY AND LIABILITIES Source: Company’s audited consolidated IFRS financial statements for the years of 2007 -2010 33

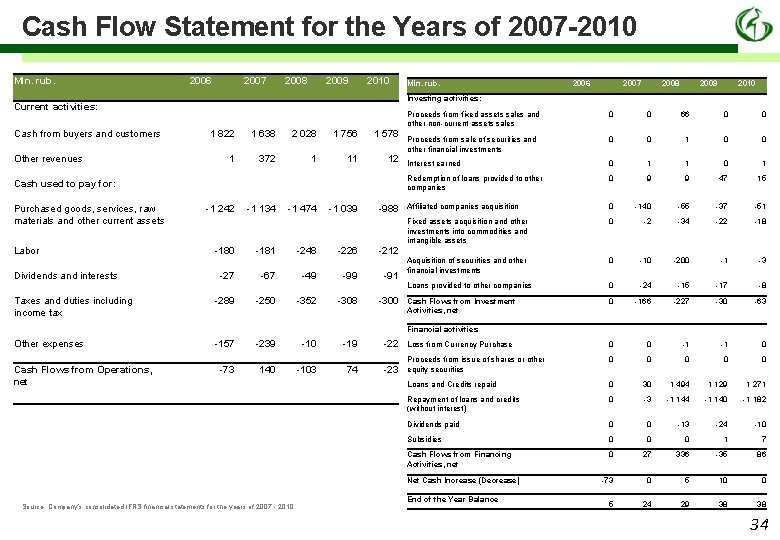
Cash Flow Statement for the Years of 2007 -2010 Mln. rub. 2006 2007 2008 2009 2010 Other revenues 1 822 1 638 2 028 1 756 1 578 1 372 1 11 12 Cash used to pay for: Purchased goods, services, raw materials and other current assets Labor Dividends and interests Taxes and duties including income tax 2006 2007 2008 2009 2010 Investing activities: Current activities: Cash from buyers and customers Mln. rub. -1 242 -1 134 -1 474 -1 039 -988 -180 -181 -248 -226 -212 -27 -67 -49 -91 -289 -250 -352 -308 -300 Proceeds from fixed assets sales and other non-current assets sales 0 0 66 0 0 Proceeds from sale of securities and other financial investments 0 0 1 0 0 Interest earned 0 1 1 0 1 Redemption of loans provided to other companies 0 9 9 47 15 Affiliated companies acquisition 0 -140 -55 -37 -51 Fixed assets acquisition and other investments into commodities and intangible assets 0 -2 -34 -22 -18 Acquisition of securities and other financial investments 0 -10 -200 -1 -3 Loans provided to other companies 0 -24 -15 -17 -8 Cash Flows from Investment Activities, net 0 -166 -227 -30 -63 Financial activities Other expenses Cash Flows from Operations, net -157 -239 -10 -19 -22 Loss from Currency Purchase 0 0 -1 -1 0 0 0 140 -103 74 -23 Proceeds from issue of shares or other equity securities 0 -73 Loans and Credits repaid 0 30 1 494 1 129 1 271 Repayment of loans and credits (without interest) 0 -3 -1 144 -1 140 -1 182 Dividends paid 0 0 -13 -24 -10 Subsidies 0 0 0 1 7 Cash Flows from Financing Activities, net 0 27 336 -35 86 -73 0 5 10 0 5 24 29 38 38 Net Cash Increase (Decrease) Source: Company’s consolidated IFRS financial statements for the years of 2007 - 2010 End of the Year Balance 34

Key Financial Parameters Forecast Appendix 2

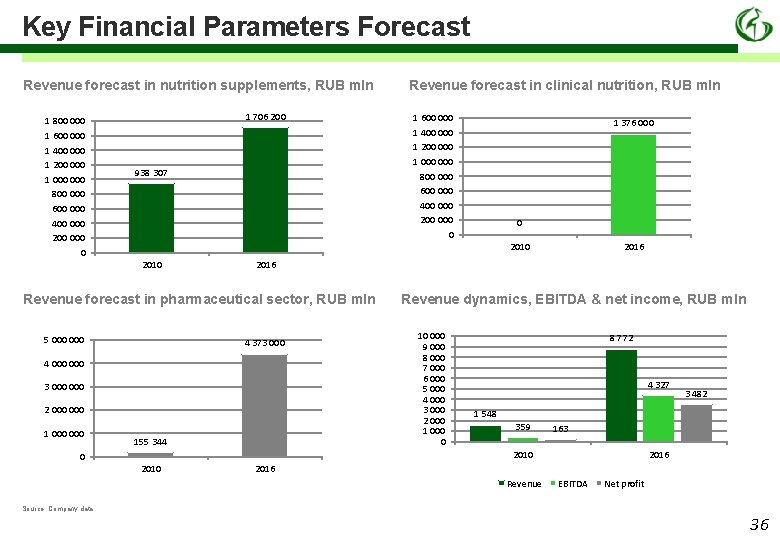
Key Financial Parameters Forecast Revenue forecast in nutrition supplements, RUB mln 1 800 000 1 600 000 1 400 000 1 200 000 1 000 800 000 600 000 400 000 200 0 1 706 200 938 307 1 600 000 1 400 000 1 200 000 1 000 800 000 600 000 400 000 200 0 1 376 000 0 2010 5 000 2016 Revenue forecast in pharmaceutical sector, RUB mln 4 373 000 4 000 3 000 2 000 1 000 Revenue forecast in clinical nutrition, RUB mln 155 344 Revenue dynamics, EBITDA & net income, RUB mln 10 000 9 000 8 000 7 000 6 000 5 000 4 000 3 000 2 000 1 000 0 8 772 4 327 1 548 359 163 2010 0 2010 3 482 2016 Revenue EBITDA Net profit Source: Company data 36

Company’s Product Portfolio Appendix 3

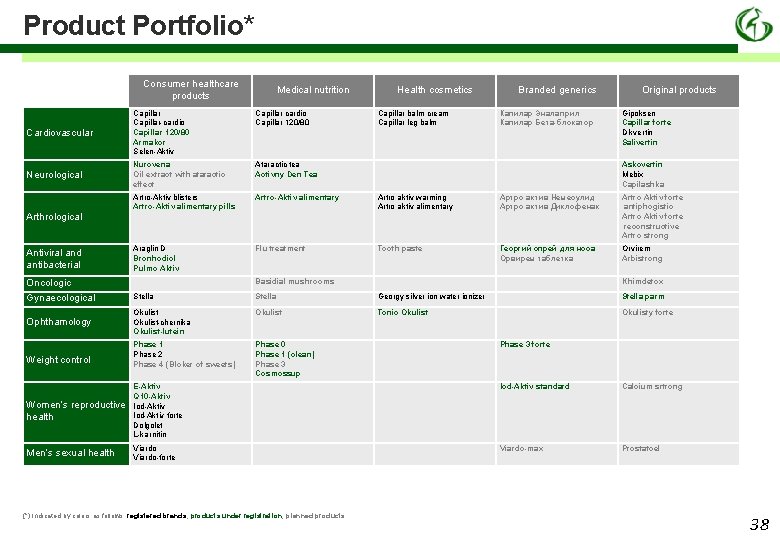
Product Portfolio* Consumer healthcare products Medical nutrition Health cosmetics Cardiovascular Capillar cardio Capillar 120/80 Neurological Nurovena Oil extract with ataractic effect Ataractic tea Activny Den Tea Artro-Aktiv blisters Artro-Aktiv alimentary pills Artro-Aktiv alimentary Artro aktiv warming Artro aktiv alimentary Артро актив Немесулид Артро актив Диклофенак Artro Aktiv forte antiphogistic Artro Aktiv forte reconstructive Artro strong Araglin D Bronhodiol Pulmo Aktiv Flu treatment Tooth paste Георгий спрей для носа Орвирем таблетка Orvirem Arbistrong Antiviral and antibacterial Капилар Эналаприл Капилар Бета-блокатор Original products Capillar-cardio Capillar 120/80 Armakor Selen-Aktiv Arthrological Capillar balm cream Capillar leg balm Branded generics Askovertin Mebix Capilashka Basidial mushrooms Oncologic Gipoksen Capillar forte Dikvertin Salivertin Khimdetox Gynaecological Stella Georgy silver ion water ionizer Stella parm Ophthamology Okulist-chernika Okulist-lutein Okulist Tonic Okulisty forte Phase 1 Phase 2 Phase 4 (Bloker of sweets) Phase 0 Phase 1 (olean) Phase 3 Cosmossup Weight control Phase 3 forte Iod-Aktiv standard Calcium srtrong Women’s reproductive health Е-Aktiv Q 10 -Aktiv Iod-Aktiv forte Dolgolet L-karnitin Men’s sexual health Viardo-forte Viardo-max Prostatcel (*) Indicated by colors as follows: registered brands, products under registration, planned products 38

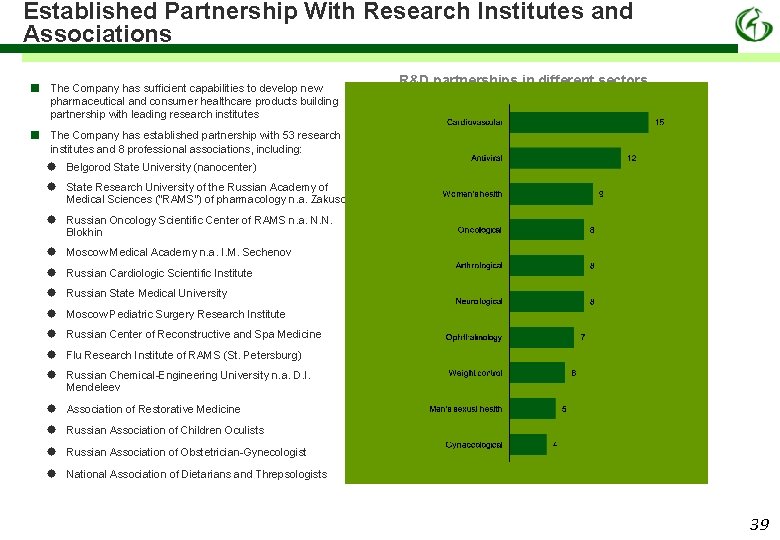
Established Partnership With Research Institutes and Associations ■ The Company has sufficient capabilities to develop new pharmaceutical and consumer healthcare products building partnership with leading research institutes ■ The Company has established partnership with 53 research institutes and 8 professional associations, including: R&D partnerships in different sectors Belgorod State University (nanocenter) State Research University of the Russian Academy of Medical Sciences (“RAMS”) of pharmacology n. a. Zakusov Russian Oncology Scientific Center of RAMS n. a. N. N. Blokhin Moscow Medical Academy n. a. I. M. Sechenov Russian Cardiologic Scientific Institute Russian State Medical University Moscow Pediatric Surgery Research Institute Russian Center of Reconstructive and Spa Medicine Flu Research Institute of RAMS (St. Petersburg) Russian Chemical-Engineering University n. a. D. I. Mendeleev Association of Restorative Medicine Russian Association of Children Oculists Russian Association of Obstetrician-Gynecologist National Association of Dietarians and Threpsologists 39

Contacts DIOD OJSC 11 A Derbenevskaya Str. Moscow 115114 Russia phone: +7 (499) 235 -2151 e-mail: investor@diod. ru IMPORTANT NOTICE Neither this document nor any copy hereof may be sent or taken or transmitted into the United States, Canada, Australia or Japan or distributed, directly or indirectly, in the United States, Canada, Australia or Japan. Any failure to comply with this restriction may constitute a violation of U. S. , Canadian, Australian or Japanese securities laws. 40