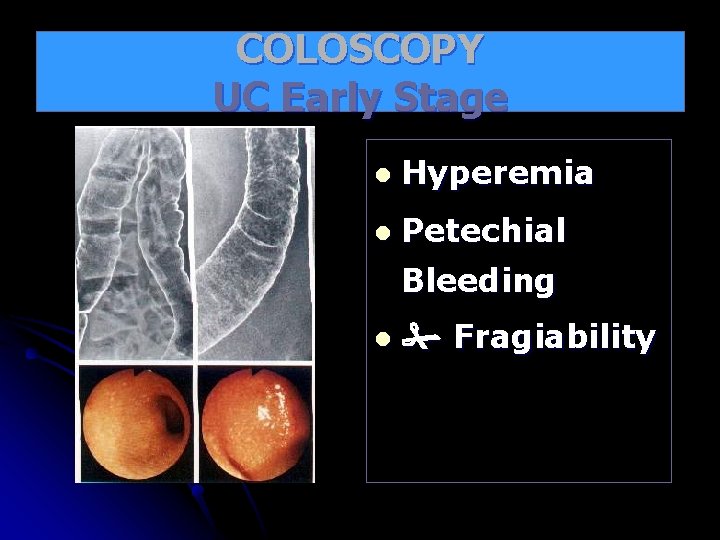
COLOSCOPY UC Early Stage l Hyperemia l Petechial









![Immunsuppressiva A. B. C. Azathiopyrin (AZT) 6 -Mercaptopurin - Cell replication ] Methotrexat (MTX) Immunsuppressiva A. B. C. Azathiopyrin (AZT) 6 -Mercaptopurin - Cell replication ] Methotrexat (MTX)](https://slidetodoc.com/presentation_image_h/d3b2e1d41410868e591e161fe565a3b2/image-29.jpg)







- Slides: 49




COLOSCOPY UC Early Stage l Hyperemia l Petechial Bleeding l Fragiability

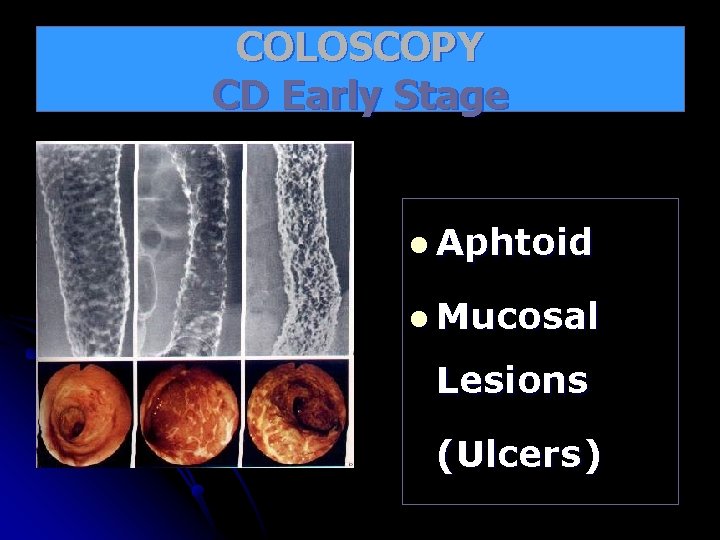
COLOSCOPY CD Early Stage l Aphtoid l Mucosal Lesions (Ulcers)

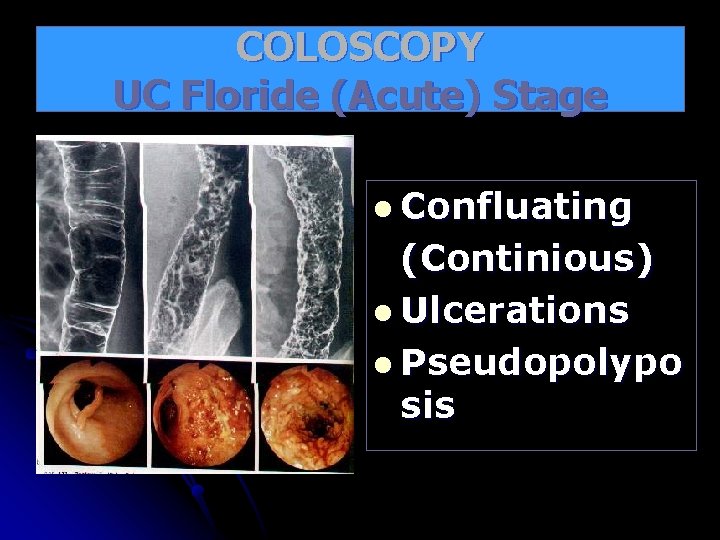
COLOSCOPY UC Floride (Acute) Stage l Confluating (Continious) l Ulcerations l Pseudopolypo sis

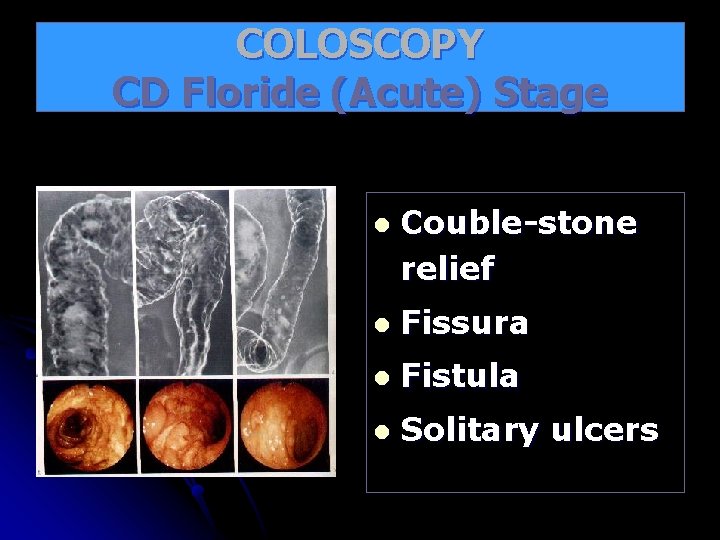
COLOSCOPY CD Floride (Acute) Stage l Couble-stone relief l Fissura l Fistula l Solitary ulcers

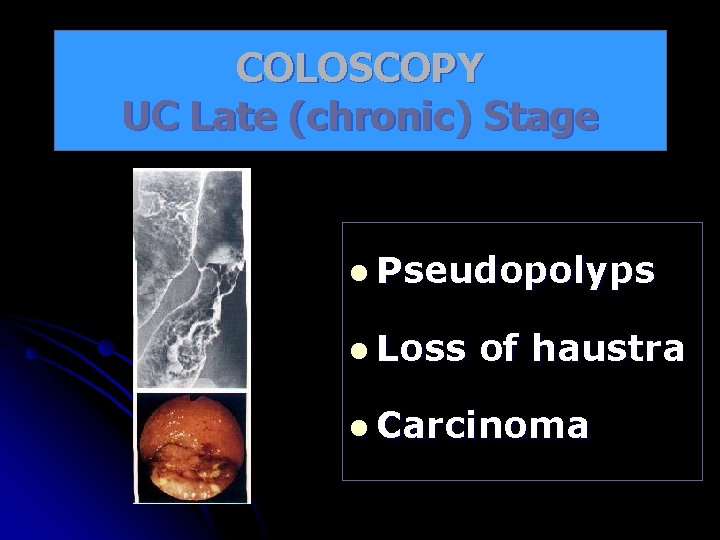
COLOSCOPY UC Late (chronic) Stage l Pseudopolyps l Loss of haustra l Carcinoma

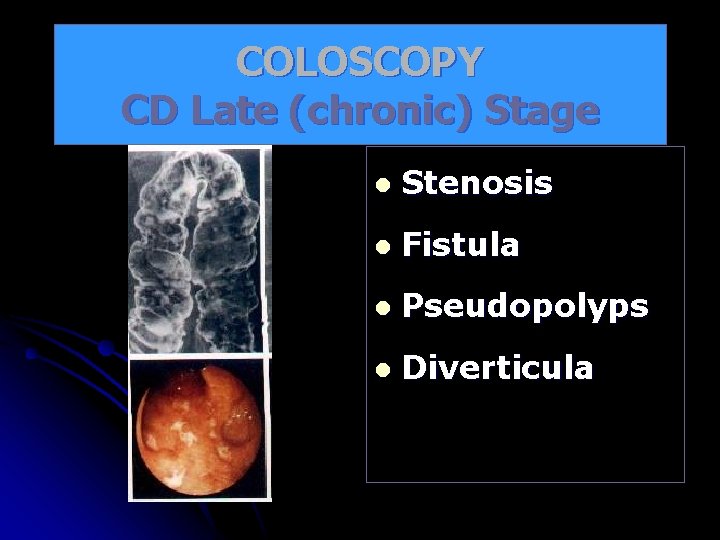
COLOSCOPY CD Late (chronic) Stage l Stenosis l Fistula l Pseudopolyps l Diverticula

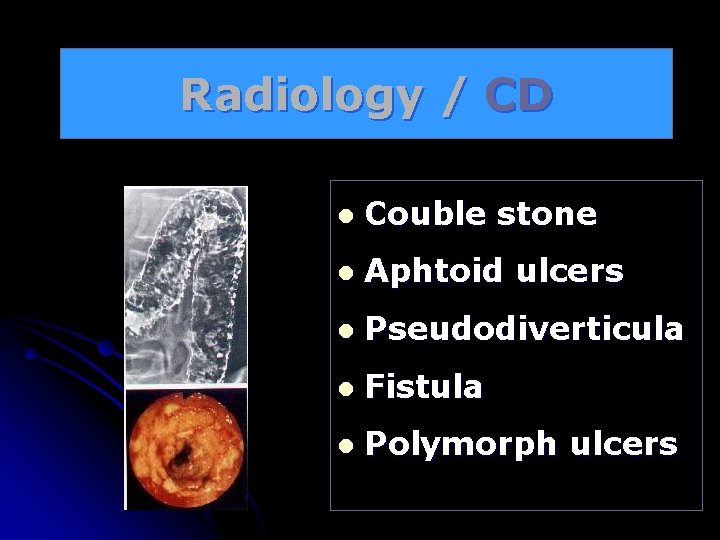
Radiology / CD l Couble stone l Aphtoid ulcers l Pseudodiverticula l Fistula l Polymorph ulcers

Activity Index Basedon - Clinical Activity - Endoscopical Activity - Histological Activity - Laboratory Activity

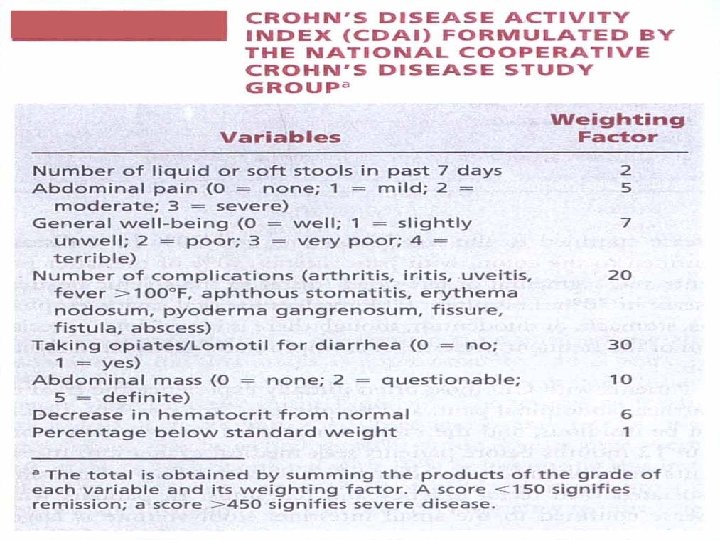
Activity Index /CD

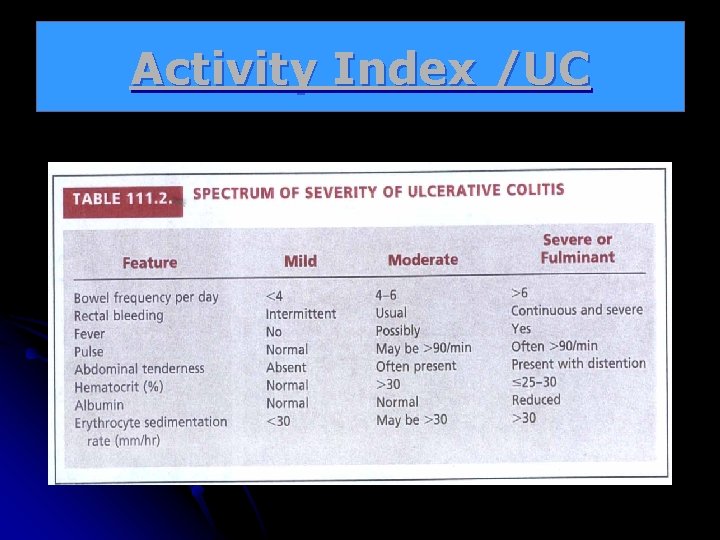
Activity Index /UC

Differential Diagnosis

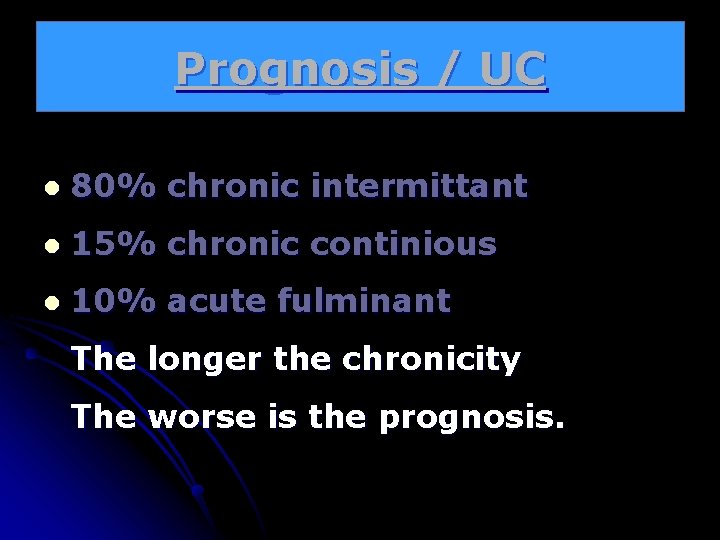
Prognosis / UC l 80% chronic intermittant l 15% chronic continious l 10% acute fulminant The longer the chronicity The worse is the prognosis.

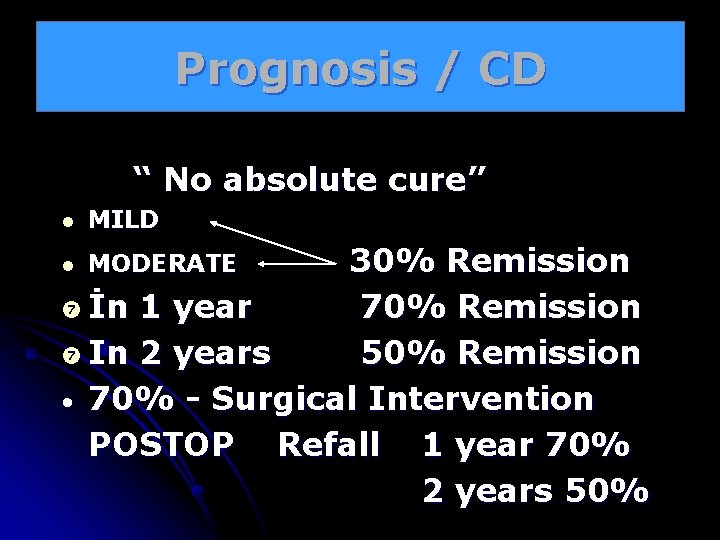
Prognosis / CD “ No absolute cure” l MILD 30% Remission İn 1 year 70% Remission In 2 years 50% Remission • 70% - Surgical Intervention POSTOP Refall 1 year 70% 2 years 50% l MODERATE

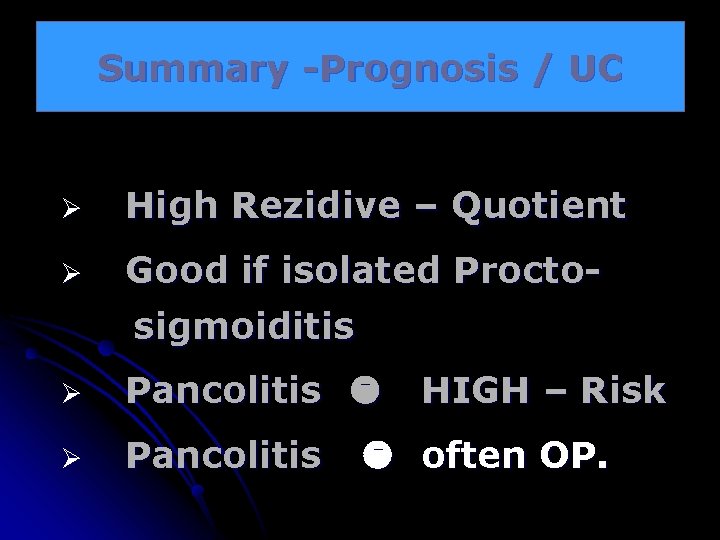
Summary -Prognosis / UC Ø High Rezidive – Quotient Ø Good if isolated Proctosigmoiditis Ø Pancolitis HIGH – Risk often OP.

Summary - Prognosis / CD Ø High – Rezidive Quotient Ø Complications OP

Goals of Therapy for IBD l Inducing remission l Maintaining remission l Restoring and maintaining nutrition l Maintaining patient’s quality of life l Surgical intervention (selection of optimal time for surgery)

Pharma-Information 1) Oral Aminosalicylates 2) Topical Aminosalicylates 3) Corticosteroids 4) Immunsuppressiva 5) Antibiotics 6) Biologic agents (anti TNF-alfa)

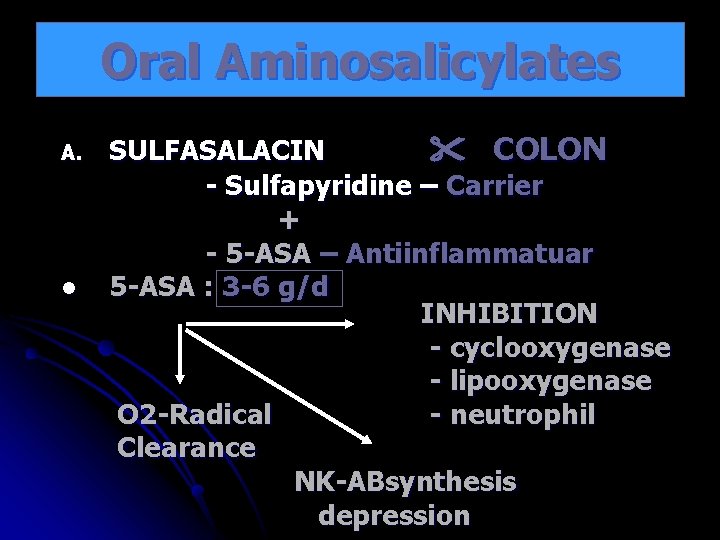
Oral Aminosalicylates A. l SULFASALACIN COLON - Sulfapyridine – Carrier + - 5 -ASA – Antiinflammatuar 5 -ASA : 3 -6 g/d INHIBITION - cyclooxygenase - lipooxygenase O 2 -Radical - neutrophil Clearance NK-ABsynthesis depression

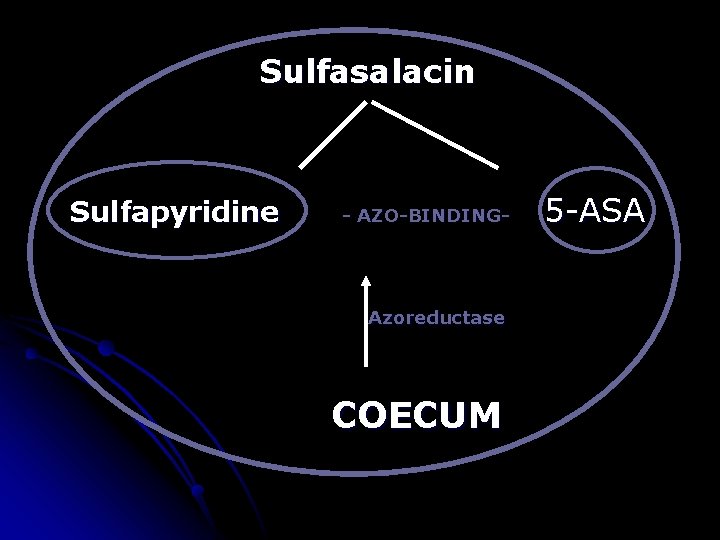
Sulfasalacin Sulfapyridine - AZO-BINDING- Azoreductase COECUM 5 -ASA

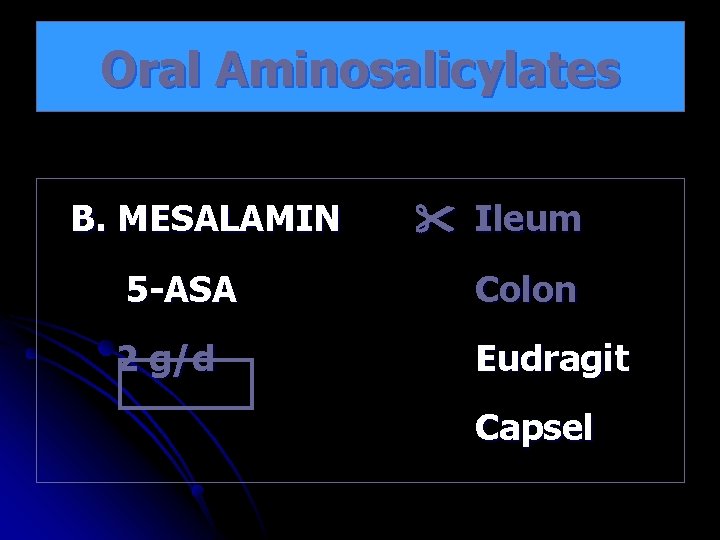
Oral Aminosalicylates B. MESALAMIN Ileum 5 -ASA Colon 2 g/d Eudragit Capsel

Topical Aminosalicylates l 5 -ASA – FOAM SUPPOSITOIRES

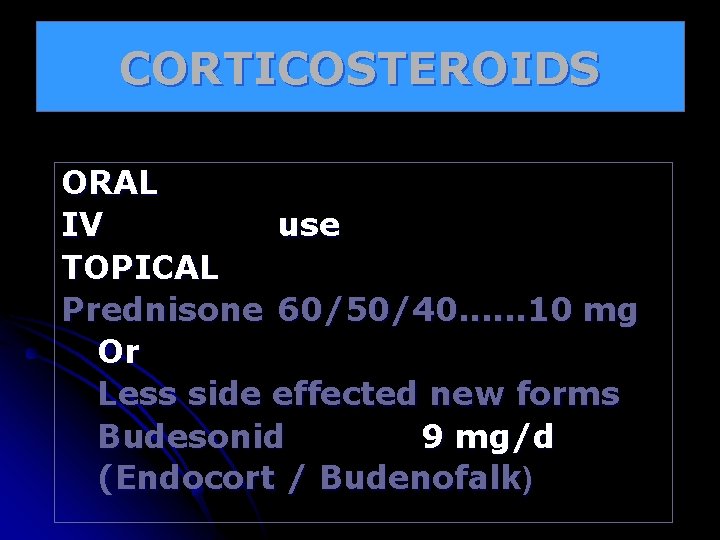
CORTICOSTEROIDS ORAL IV use TOPICAL Prednisone 60/50/40. . . 10 mg Or Less side effected new forms Budesonid 9 mg/d (Endocort / Budenofalk)

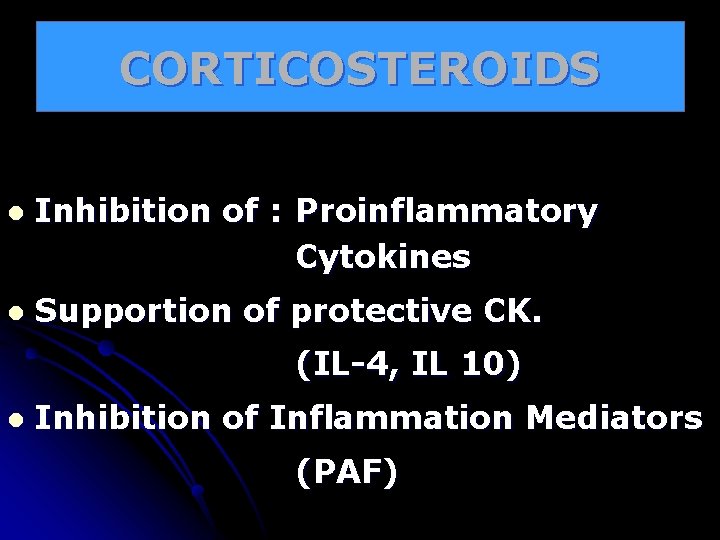
CORTICOSTEROIDS l Inhibition of : Proinflammatory Cytokines l Supportion of protective CK. (IL-4, IL 10) l Inhibition of Inflammation Mediators (PAF)

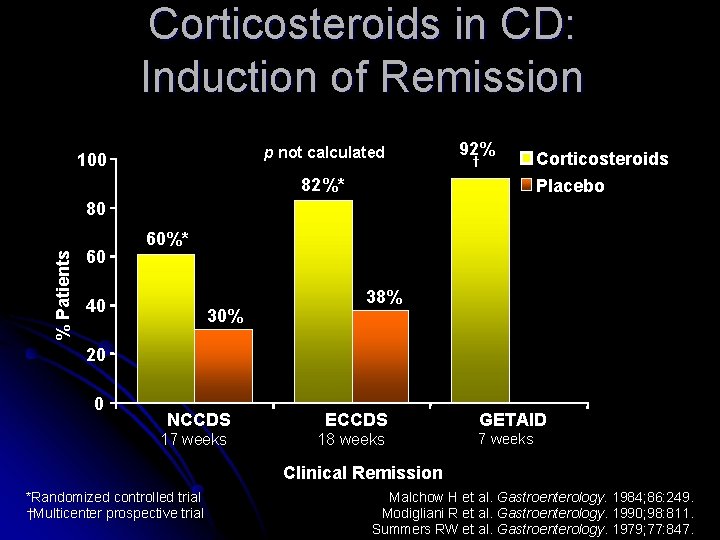
Corticosteroids in CD: Induction of Remission p not calculated 100 92% † 82%* Corticosteroids Placebo % Patients 80 60 60%* 40 30% 38% 20 0 NCCDS ECCDS 17 weeks 18 weeks GETAID 7 weeks Clinical Remission *Randomized controlled trial †Multicenter prospective trial Malchow H et al. Gastroenterology. 1984; 86: 249. Modigliani R et al. Gastroenterology. 1990; 98: 811. Summers RW et al. Gastroenterology. 1979; 77: 847.

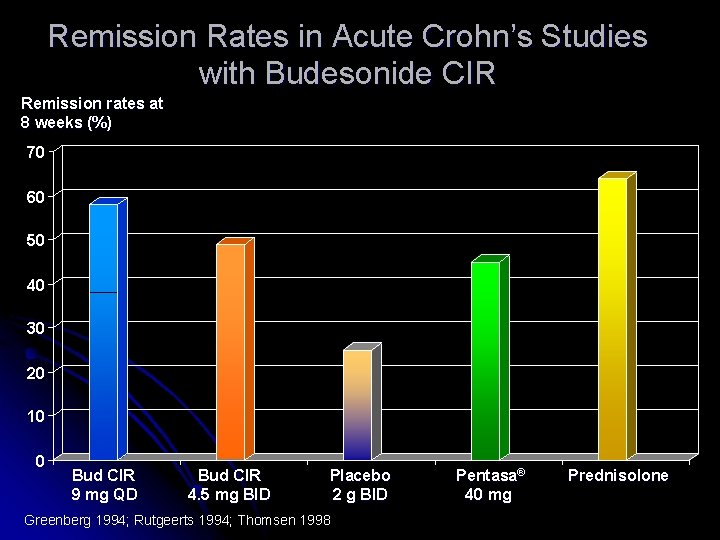
Remission Rates in Acute Crohn’s Studies with Budesonide CIR Remission rates at 8 weeks (%) 70 60 50 40 30 20 10 0 Bud CIR 9 mg QD Bud CIR 4. 5 mg BID Placebo 2 g BID Greenberg 1994; Rutgeerts 1994; Thomsen 1998 Pentasa® 40 mg Prednisolone
![Immunsuppressiva A B C Azathiopyrin AZT 6 Mercaptopurin Cell replication Methotrexat MTX Immunsuppressiva A. B. C. Azathiopyrin (AZT) 6 -Mercaptopurin - Cell replication ] Methotrexat (MTX)](https://slidetodoc.com/presentation_image_h/d3b2e1d41410868e591e161fe565a3b2/image-29.jpg)
Immunsuppressiva A. B. C. Azathiopyrin (AZT) 6 -Mercaptopurin - Cell replication ] Methotrexat (MTX) - Antimetabolite - Inhibition of Dihydrofolacid reductase + Lymphocytic Proliferation Cyclosporin - Immunmodulater - T-Cell depression

Antibiotics • Metronidazol

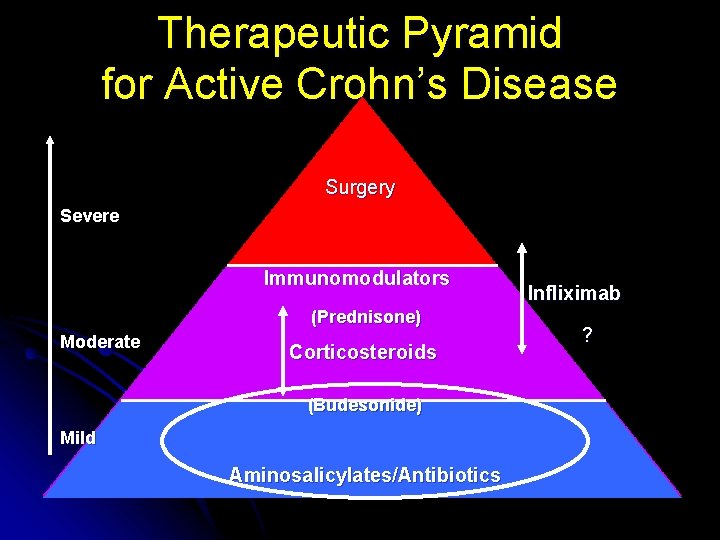
Therapeutic Pyramid for Active Crohn’s Disease Surgery Severe Immunomodulators (Prednisone) Moderate Corticosteroids (Budesonide) Mild Aminosalicylates/Antibiotics Infliximab ?

Outcomes for Mild-Moderate Disease Aminosalicylate Response 40 -50% Antibiotic (Colonic Disease) Response 40 -50% Budesonide (Ileum-Right Colon) Response 50 -65% Placebo Response 30 -40%

Biologic agents • İnfliximap • adaluminap

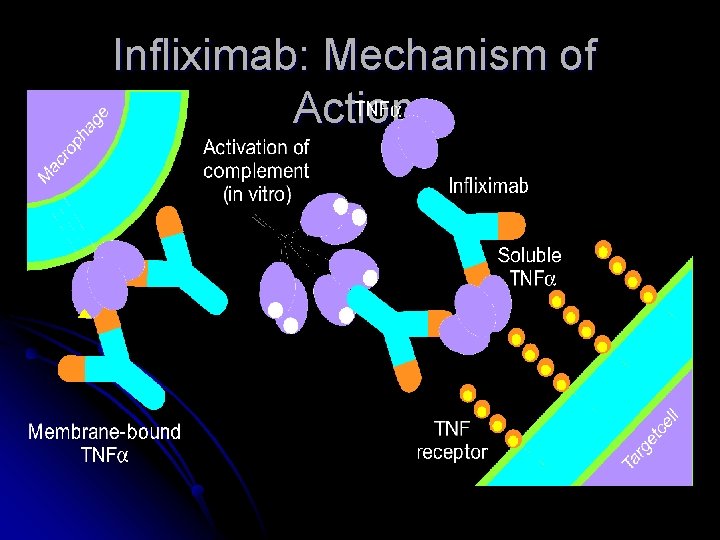
Infliximab: Mechanism of Action

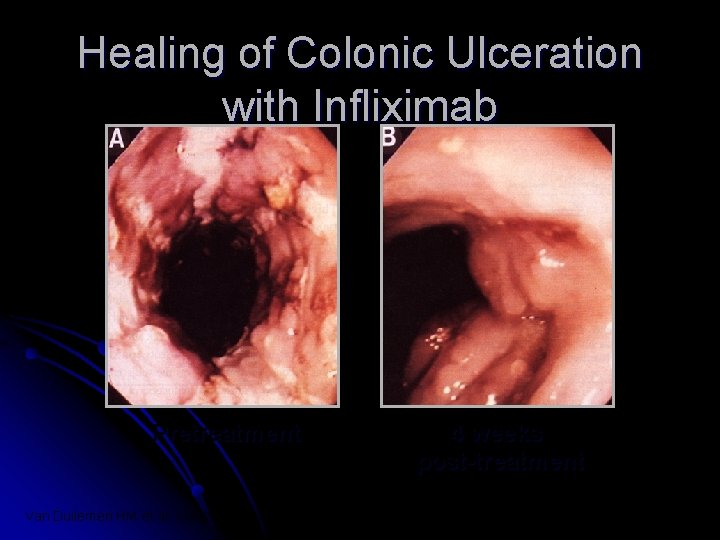
Healing of Colonic Ulceration with Infliximab Pretreatment Van Dullemen HM et al. Gastroenterology 1995; 109: 129 -135 4 weeks post-treatment

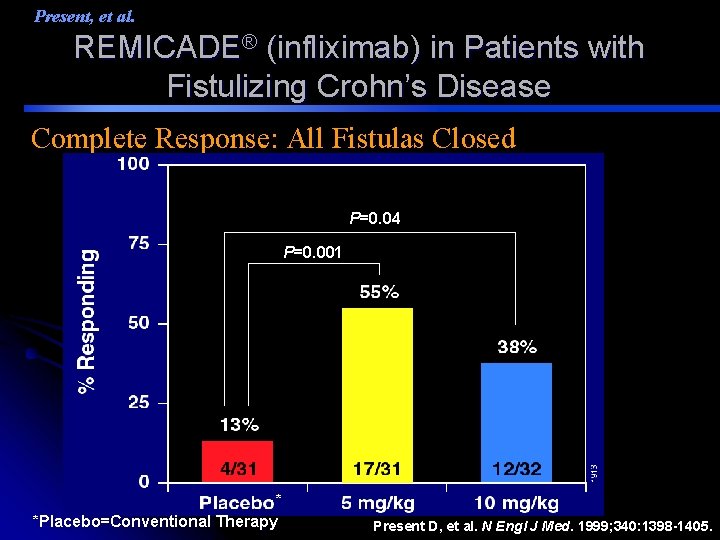
Present, et al. REMICADE® (infliximab) in Patients with Fistulizing Crohn’s Disease Complete Response: All Fistulas Closed P=0. 04 P=0. 001 * *Placebo=Conventional Therapy Present D, et al. N Engl J Med. 1999; 340: 1398 -1405.

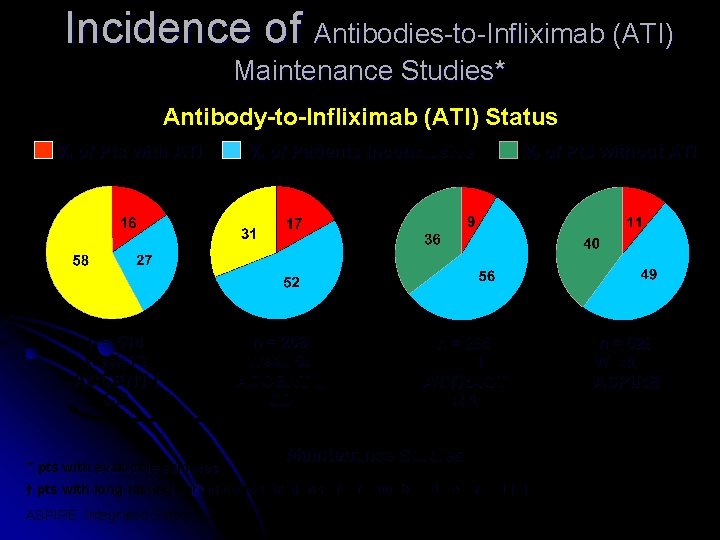
Incidence of Antibodies-to-Infliximab (ATI) Maintenance Studies* Antibody-to-Infliximab (ATI) Status % of Pts with ATI n = 514 Week 72 ACCENT I CD * pts with evaluable samples % of Patients Inconclusive† n = 258 Week 54 ACCENT II CD % of Pts without ATI n = 295 Week 102 ATTRACT RA Maintenance Studies † pts with long-lasting serum concentrations of infliximab and never ATI (+) ASPIRE: Integrated Safety Summary, Sep. 18, 2003 n = 629 Week 54 ASPIRE RA

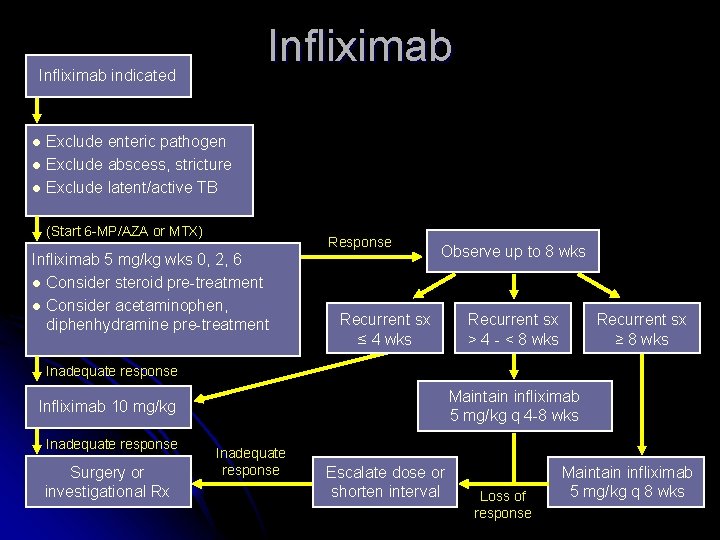
Infliximab indicated Exclude enteric pathogen l Exclude abscess, stricture l Exclude latent/active TB l (Start 6 -MP/AZA or MTX) Response Infliximab 5 mg/kg wks 0, 2, 6 l Consider steroid pre-treatment l Consider acetaminophen, diphenhydramine pre-treatment Observe up to 8 wks Recurrent sx ≤ 4 wks Recurrent sx > 4 - < 8 wks Recurrent sx ≥ 8 wks Inadequate response Maintain infliximab 5 mg/kg q 4 -8 wks Infliximab 10 mg/kg Inadequate response Surgery or investigational Rx Inadequate response Escalate dose or shorten interval Loss of response Maintain infliximab 5 mg/kg q 8 wks

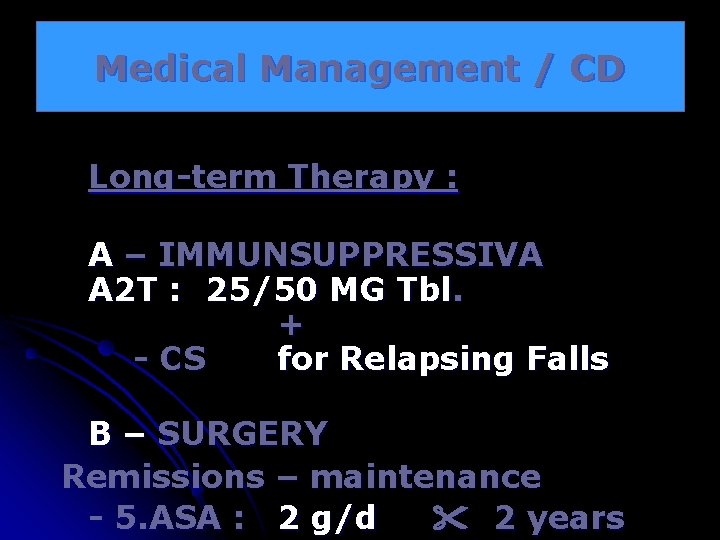
Medical Management / CD Long-term Therapy : A – IMMUNSUPPRESSIVA A 2 T : 25/50 MG Tbl. + - CS for Relapsing Falls B – SURGERY Remissions – maintenance - 5. ASA : 2 g/d 2 years

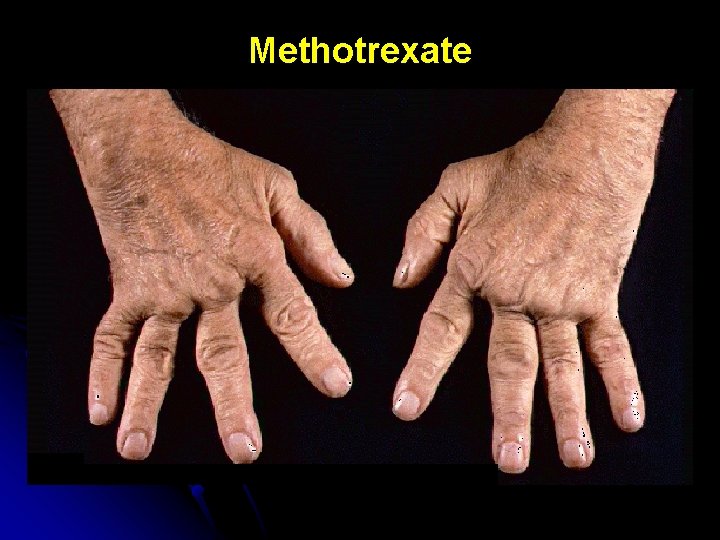
Methotrexate

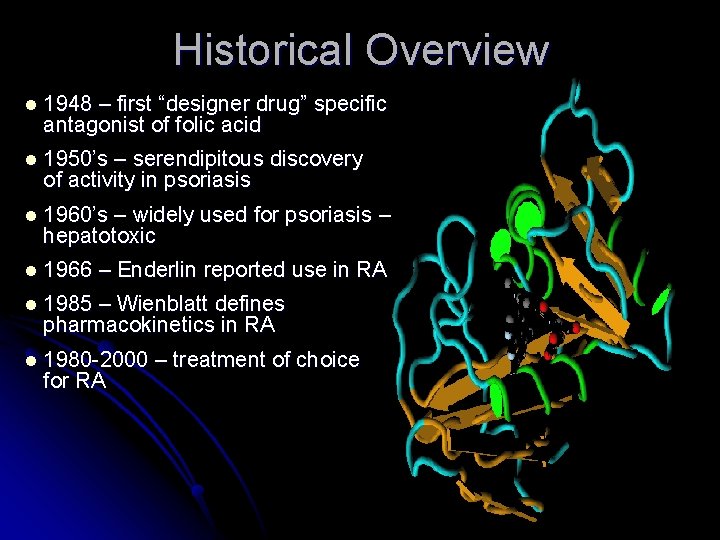
Historical Overview l 1948 – first “designer drug” specific antagonist of folic acid l 1950’s – serendipitous discovery of activity in psoriasis l 1960’s – widely used for psoriasis – hepatotoxic l 1966 – Enderlin reported use in RA l 1985 – Wienblatt defines pharmacokinetics in RA l 1980 -2000 for RA – treatment of choice

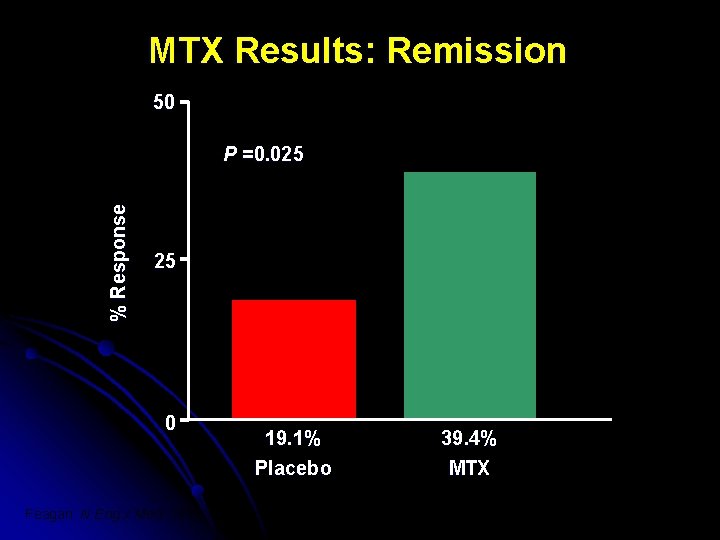
MTX Results: Remission 50 % Response P =0. 025 25 0 19. 1% Placebo Feagan. N Eng J Med. 1995; 332(5): 292 -7 39. 4% MTX

Methotrexate in IBD: Toxicity l Major l Minor l Hepatic l Gastrointestinal l Myelosuppressive l Alopecia-inductive l Pulmonary l Allergic l Fertility-related l Neurologic l Teratogenic l Enteritic/colitic Egan LJ, Sandborn WJ. Mayo Clin Proc 1996; 71: 69 -80

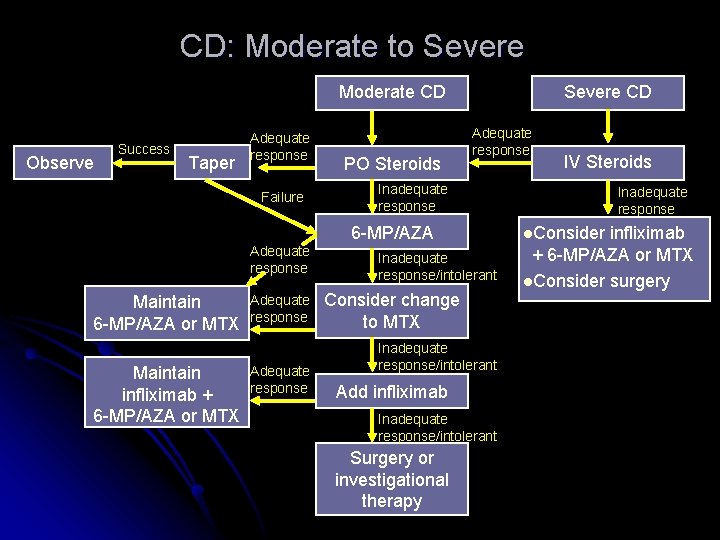
CD: Moderate to Severe Moderate CD Observe Success Taper Adequate response Failure PO Steroids Severe CD Adequate response Inadequate response 6 -MP/AZA Adequate response Maintain 6 -MP/AZA or MTX Maintain infliximab + 6 -MP/AZA or MTX Adequate response IV Steroids Inadequate response/intolerant Consider change to MTX Inadequate response/intolerant Add infliximab Inadequate response/intolerant Surgery or investigational therapy Inadequate response l. Consider infliximab + 6 -MP/AZA or MTX l. Consider surgery

Medical Management / UC l Refractory States or Chronic active Forms l Immunsuppressiva A 2 T : +? Cs OP Proctocolectomy (= Definitive Cure)

Ulcerative Colitis l Remissions l 5 -ASA – Maintenance 2 gr/d

OP – Indications / CD Bleeding l Ileus l Stenosis l Fistula l Carcinom l Perforation l Abcess l

OP – Indications / UC l Toxic Megacolon l Perforation l Severe Bleeding

 Petechial hemorrhages
Petechial hemorrhages Petechial hemorrhages
Petechial hemorrhages Hemodynamic disorders
Hemodynamic disorders Reactive hyperemia
Reactive hyperemia Blood circulation
Blood circulation Endothelix
Endothelix Rebound hyperemia
Rebound hyperemia Difference between hyperemia and congestion
Difference between hyperemia and congestion Early cpr and early defibrillation can: *
Early cpr and early defibrillation can: * Early stage investigator nih
Early stage investigator nih Dentinogenesis
Dentinogenesis Characteristics of early adulthood
Characteristics of early adulthood Early years foundation stage pack
Early years foundation stage pack Early adulthood physical changes
Early adulthood physical changes Upstage right
Upstage right Stage one denial stage two
Stage one denial stage two Stage right stage left diagram
Stage right stage left diagram Arena stage disadvantages
Arena stage disadvantages Single stage tender
Single stage tender Downstage and upstage
Downstage and upstage Early christian art
Early christian art Early victorian age
Early victorian age Tanner scale
Tanner scale Doncaster early help
Doncaster early help Smyth model of reflection
Smyth model of reflection National breast and cervical cancer early detection program
National breast and cervical cancer early detection program Second step social emotional skills for early learning
Second step social emotional skills for early learning Early learning for everyone
Early learning for everyone Dot symbol
Dot symbol How did early humans live
How did early humans live Sandalwood high school bell schedule
Sandalwood high school bell schedule Sri lanka tsunami warning system
Sri lanka tsunami warning system Brigance inventory of early development
Brigance inventory of early development Omebase
Omebase Early mesial shift
Early mesial shift 1 week darkening areola early pregnancy pictures
1 week darkening areola early pregnancy pictures Early years summit
Early years summit Early and high renaissance
Early and high renaissance Answer
Answer Efq
Efq Early adulthood milestones
Early adulthood milestones Early and late complications of blood transfusion
Early and late complications of blood transfusion Lesson 1 early civilizations
Lesson 1 early civilizations Byzantine
Byzantine Ohsc
Ohsc Quiz 1: the early missionary journeys
Quiz 1: the early missionary journeys Early childhood education barbados
Early childhood education barbados Eminem early childhood
Eminem early childhood Early learning for everyone
Early learning for everyone Section 17-2 earth's early history
Section 17-2 earth's early history