Cloud Physics Cloud formation and precipitation processes Guest







- Slides: 26

Cloud Physics Cloud formation and precipitation processes Guest lecture by Prof. Kathleen Schiro 12 October 2020

Cloud Droplet Formation • Review of how condensation occurs • Curvature effect • Cloud condensation nuclei • Solute effect

3. 3 x 105 J/kg 2. 5 x 106 J/kg Latent heat (L) of water phase changes

Condensation and droplet formation Condensation occurs when e = es. One key mechanism for decreasing temperature is lifting the parcel of air to higher elevation. As a parcel of air is lifted to higher elevations, it becomes cooler (adiabatic expansion) and es decreases. The term lifting condensation level (LCL) is often used to describe this elevation. Broadly speaking, there are 3 types of lifting mechanisms: Orographic lifting: When air is forced to go over mountains Frontal surface lifting: When warm, moist air is forced above cooler air Convection: Warm, moist air rises because it is less dense than its surroundings *Condensation can also occur due to radiative cooling, horizontal advection, mixing

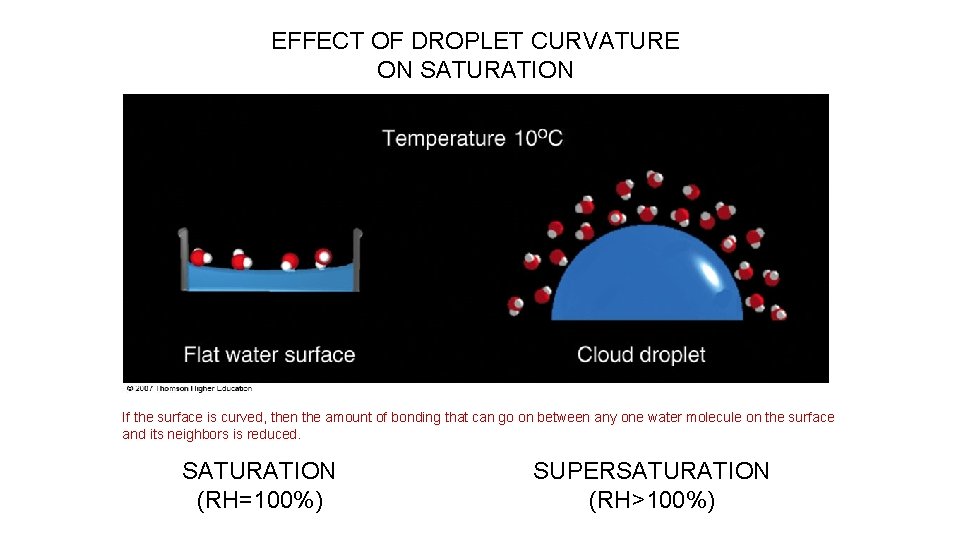
EFFECT OF DROPLET CURVATURE ON SATURATION If the surface is curved, then the amount of bonding that can go on between any one water molecule on the surface and its neighbors is reduced. SATURATION (RH=100%) SUPERSATURATION (RH>100%)

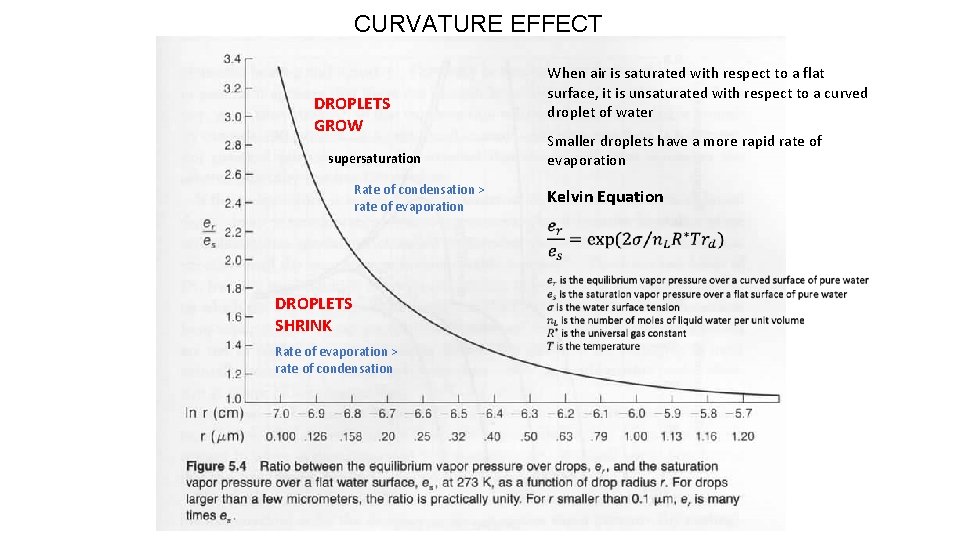
CURVATURE EFFECT DROPLETS GROW supersaturation Rate of condensation > rate of evaporation DROPLETS SHRINK Rate of evaporation > rate of condensation When air is saturated with respect to a flat surface, it is unsaturated with respect to a curved droplet of water Smaller droplets have a more rapid rate of evaporation Kelvin Equation

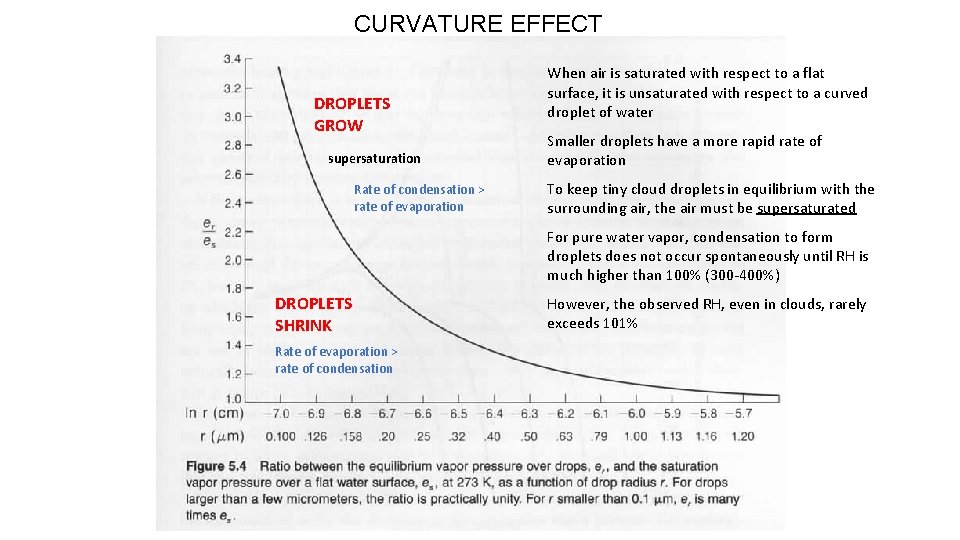
CURVATURE EFFECT DROPLETS GROW supersaturation Rate of condensation > rate of evaporation When air is saturated with respect to a flat surface, it is unsaturated with respect to a curved droplet of water Smaller droplets have a more rapid rate of evaporation To keep tiny cloud droplets in equilibrium with the surrounding air, the air must be supersaturated For pure water vapor, condensation to form droplets does not occur spontaneously until RH is much higher than 100% (300 -400%) DROPLETS SHRINK Rate of evaporation > rate of condensation However, the observed RH, even in clouds, rarely exceeds 101%

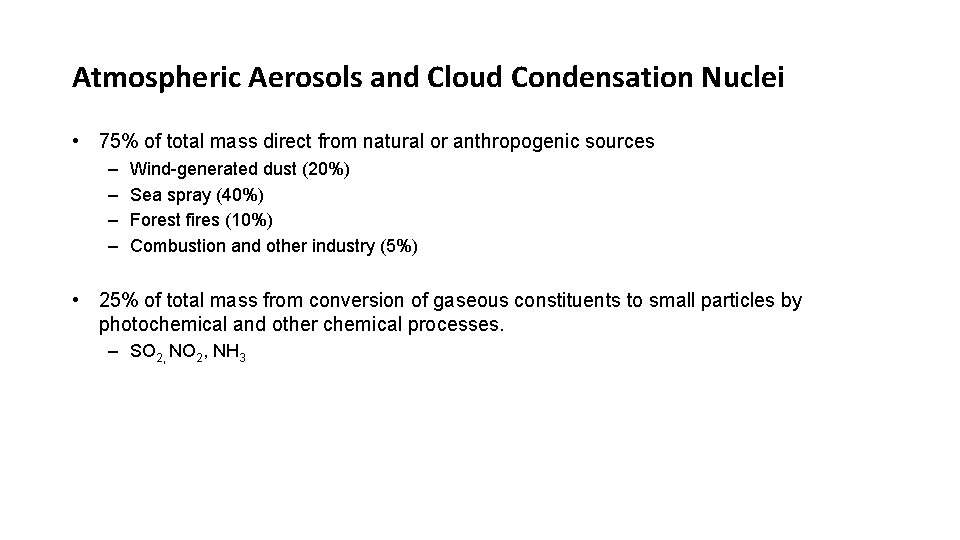
Atmospheric Aerosols and Cloud Condensation Nuclei • 75% of total mass direct from natural or anthropogenic sources – – Wind-generated dust (20%) Sea spray (40%) Forest fires (10%) Combustion and other industry (5%) • 25% of total mass from conversion of gaseous constituents to small particles by photochemical and other chemical processes. – SO 2, NH 3

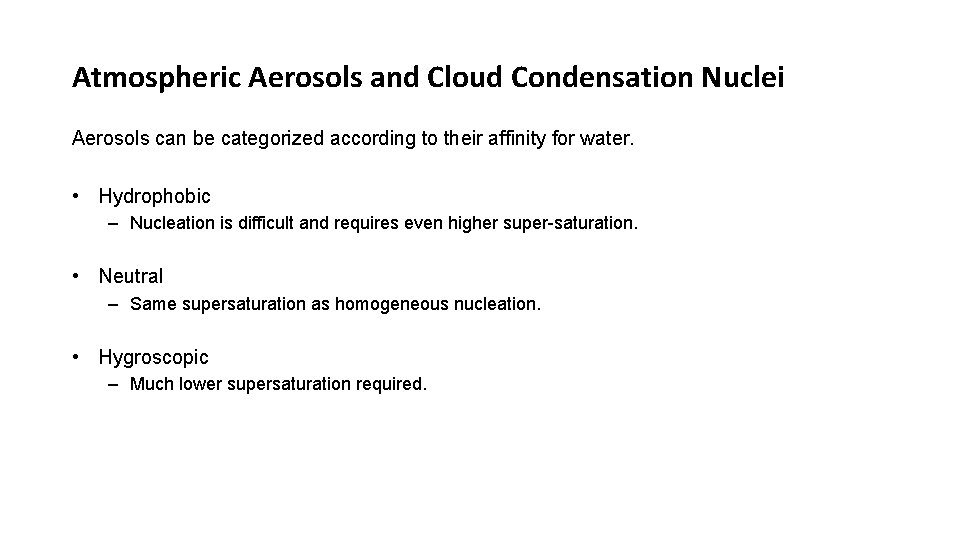
Atmospheric Aerosols and Cloud Condensation Nuclei Aerosols can be categorized according to their affinity for water. • Hydrophobic – Nucleation is difficult and requires even higher super-saturation. • Neutral – Same supersaturation as homogeneous nucleation. • Hygroscopic – Much lower supersaturation required.

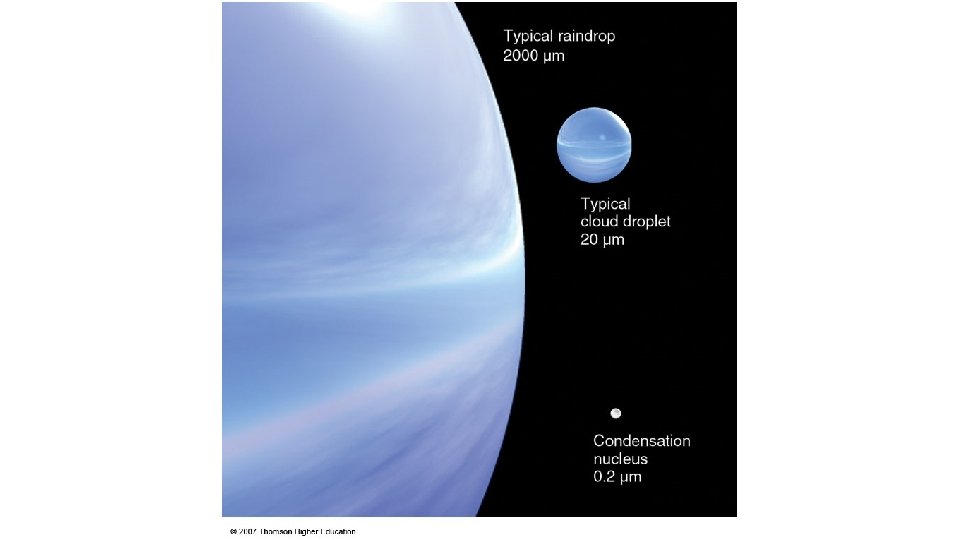
Atmospheric Aerosols and Cloud Condensation Nuclei (CCN) are hygroscopic aerosols that are necessary for cloud formation • CCN provide a surface to encourage condensation of water vapor molecules • If vapor pressure of solute is less than that of the solvent, the vapor pressure of solution (droplet) is reduced. – Bonding with solute makes it more difficult for water molecules to evaporate – A solution droplet can be in equilibrium at a much lower supersaturation than a pure water droplet of the same size. • There about 10 times more CCN in continental than maritime air masses

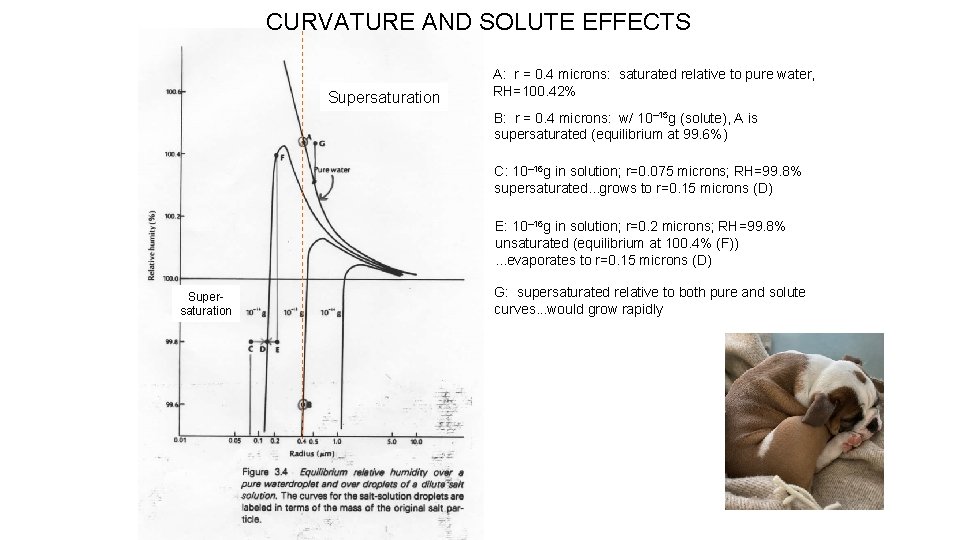
CURVATURE AND SOLUTE EFFECTS Supersaturation A: r = 0. 4 microns: saturated relative to pure water, RH=100. 42% B: r = 0. 4 microns: w/ 10– 15 g (solute), A is supersaturated (equilibrium at 99. 6%) C: 10– 16 g in solution; r=0. 075 microns; RH=99. 8% supersaturated. . . grows to r=0. 15 microns (D) E: 10– 16 g in solution; r=0. 2 microns; RH=99. 8% unsaturated (equilibrium at 100. 4% (F)). . . evaporates to r=0. 15 microns (D) Supersaturation G: supersaturated relative to both pure and solute curves. . . would grow rapidly


Condensation is a necessary but insufficient condition for precipitation formation.

Precipitation Formation • Collision and Coalescence • Bergeron process • Hydrometeor types (rain, snow, sleet, freezing rain, hail)

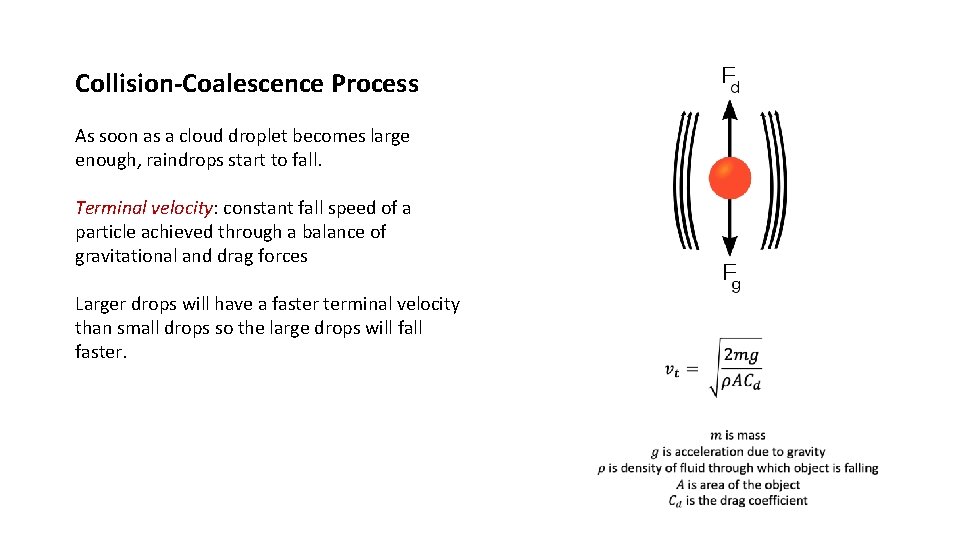
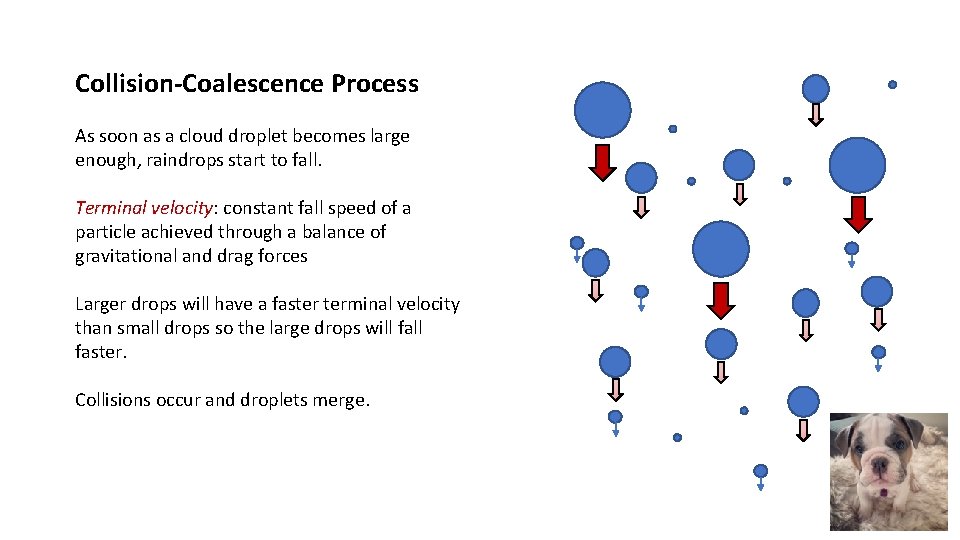
Collision-Coalescence Process As soon as a cloud droplet becomes large enough, raindrops start to fall. Terminal velocity: constant fall speed of a particle achieved through a balance of gravitational and drag forces Larger drops will have a faster terminal velocity than small drops so the large drops will faster.

Collision-Coalescence Process As soon as a cloud droplet becomes large enough, raindrops start to fall. Terminal velocity: constant fall speed of a particle achieved through a balance of gravitational and drag forces Larger drops will have a faster terminal velocity than small drops so the large drops will faster. Collisions occur and droplets merge.

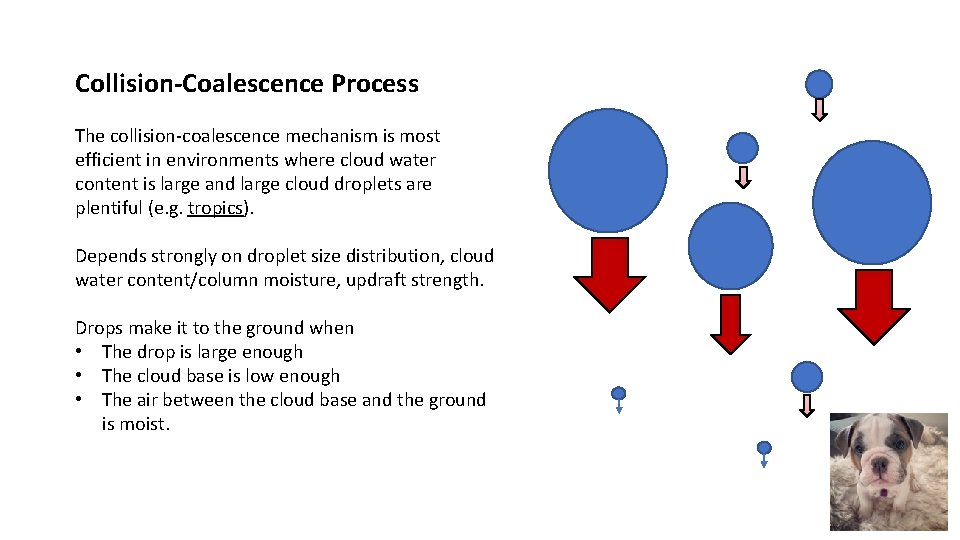
Collision-Coalescence Process The collision-coalescence mechanism is most efficient in environments where cloud water content is large and large cloud droplets are plentiful (e. g. tropics). Depends strongly on droplet size distribution, cloud water content/column moisture, updraft strength. Drops make it to the ground when • The drop is large enough • The cloud base is low enough • The air between the cloud base and the ground is moist.

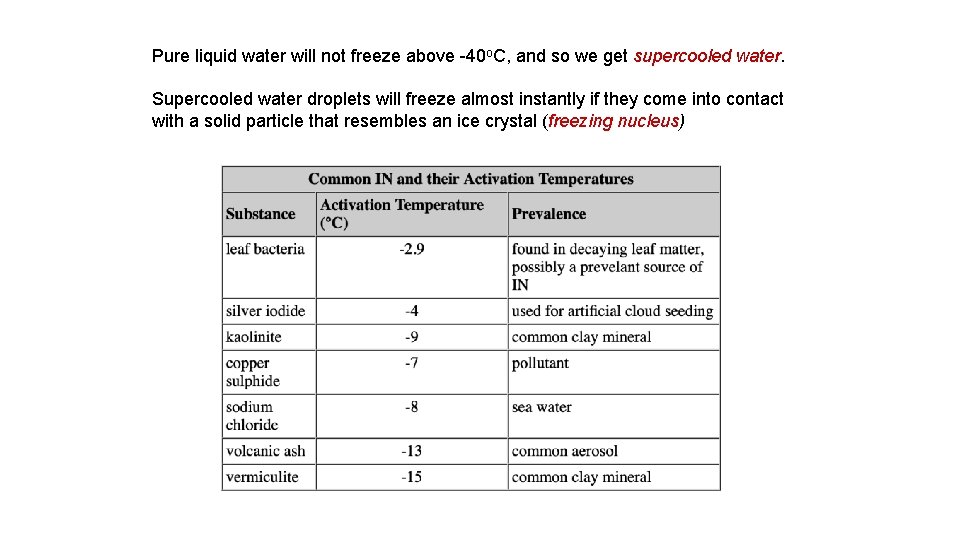
Pure liquid water will not freeze above -40 o. C, and so we get supercooled water. Supercooled water droplets will freeze almost instantly if they come into contact with a solid particle that resembles an ice crystal (freezing nucleus)

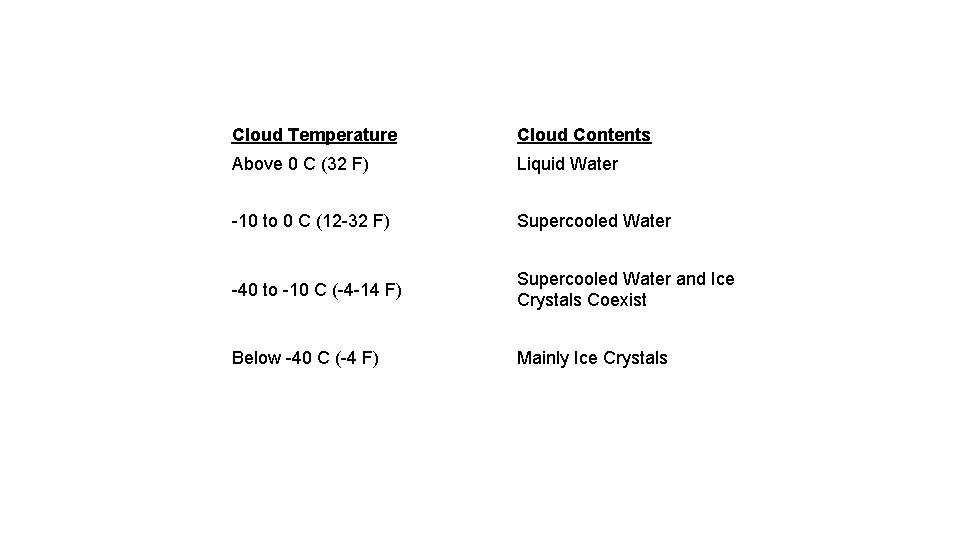
Cloud Temperature Cloud Contents Above 0 C (32 F) Liquid Water -10 to 0 C (12 -32 F) Supercooled Water -40 to -10 C (-4 -14 F) Supercooled Water and Ice Crystals Coexist Below -40 C (-4 F) Mainly Ice Crystals

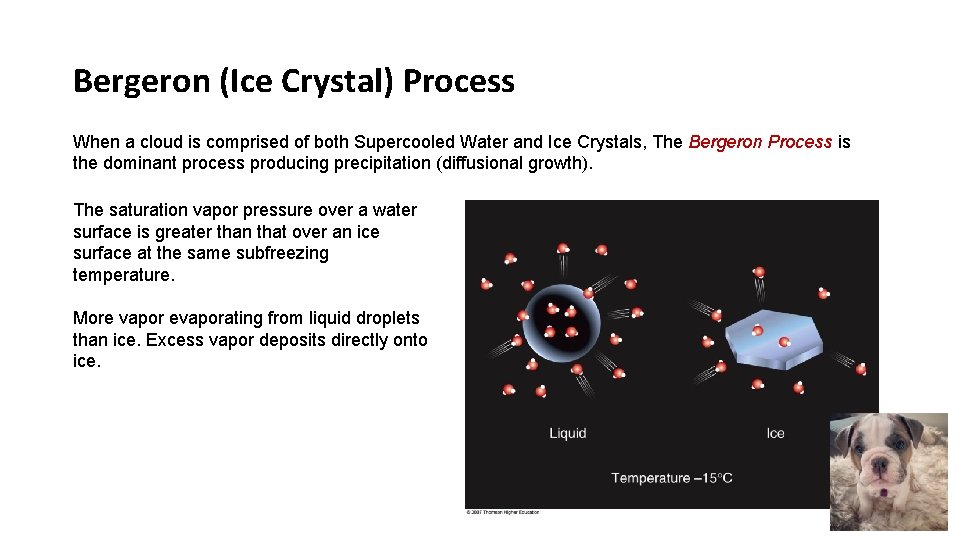
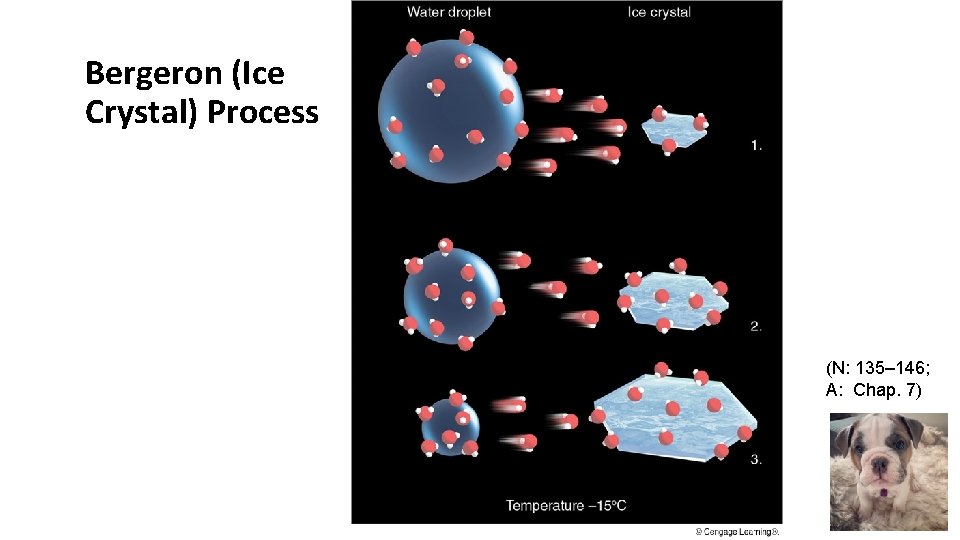
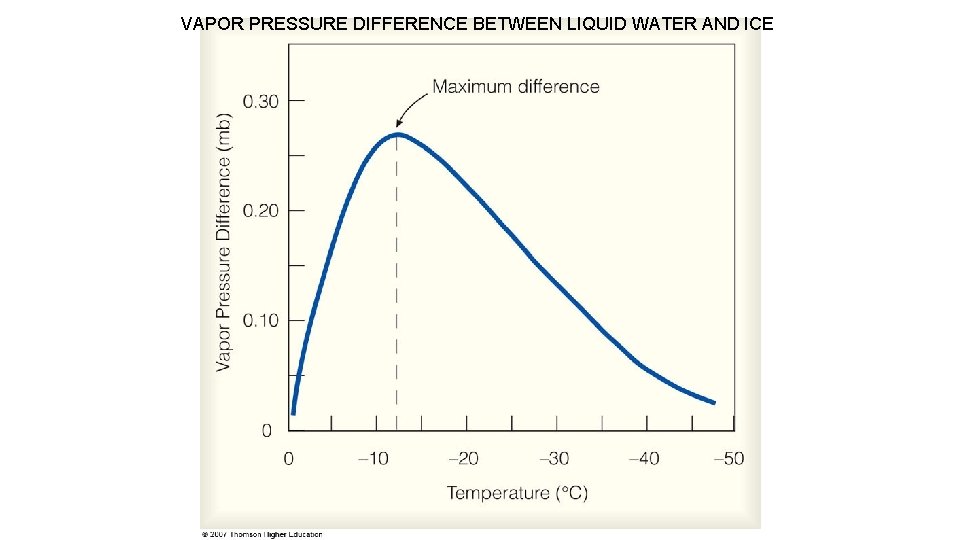
Bergeron (Ice Crystal) Process When a cloud is comprised of both Supercooled Water and Ice Crystals, The Bergeron Process is the dominant process producing precipitation (diffusional growth). The saturation vapor pressure over a water surface is greater than that over an ice surface at the same subfreezing temperature. More vapor evaporating from liquid droplets than ice. Excess vapor deposits directly onto ice.

Bergeron (Ice Crystal) Process (N: 135– 146; A: Chap. 7)


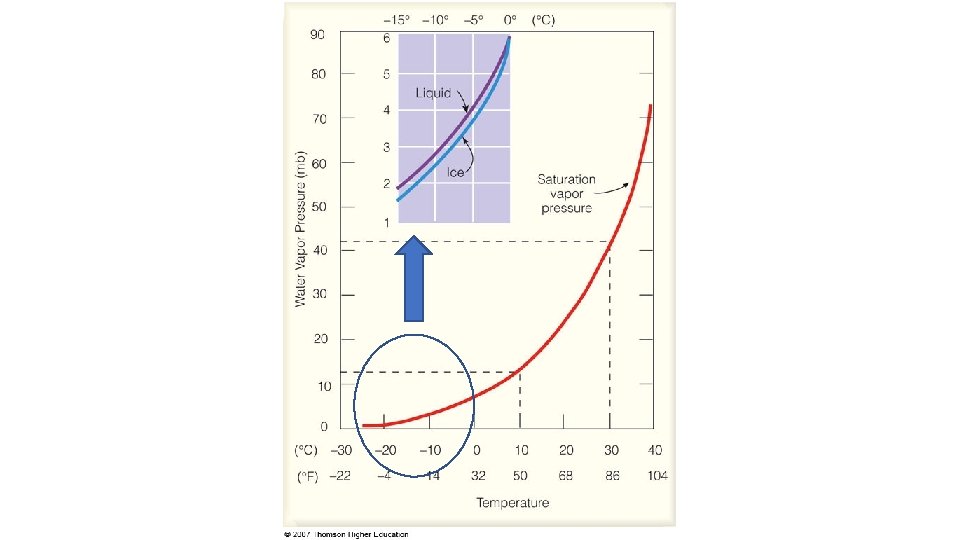
VAPOR PRESSURE DIFFERENCE BETWEEN LIQUID WATER AND ICE

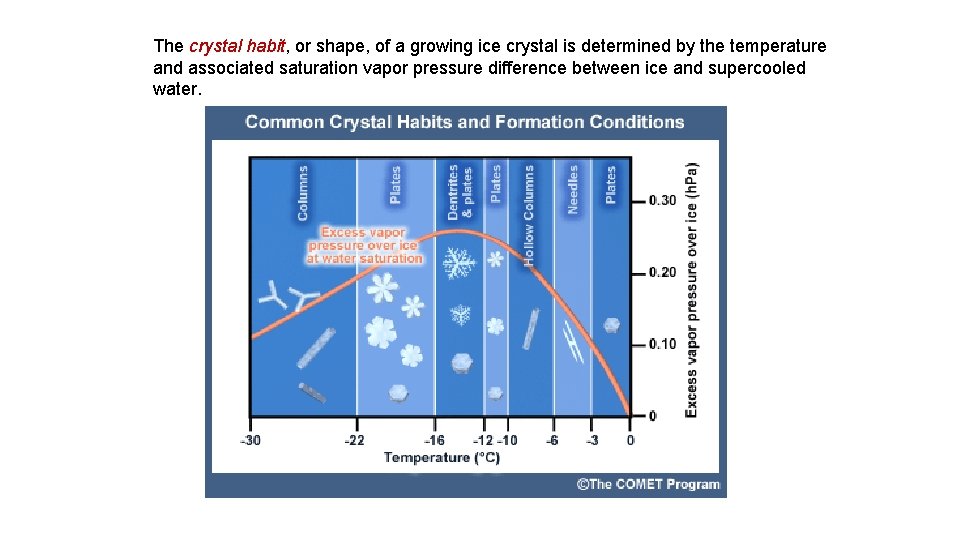
The crystal habit, or shape, of a growing ice crystal is determined by the temperature and associated saturation vapor pressure difference between ice and supercooled water.

Snowflakes result from the aggregation of ice crystals created by the Bergeron process. Snowflakes have a very low density, and hence fall relatively slowly through the air (≈ 1 m s− 1). Graupel consists of ice crystals or snowflakes which have encountered additional supercooled liquid. When such droplets impact these particles, they freeze instantly, a process called riming. Riming tends to fill in the gaps between crystals, resulting in particles with densities higher than that of snowflakes, but less than that of pure ice. Pbs. com wikipedia. com Hail is produced when graupel encounters especially high concentrations of supercooled water. Formed in cumulonimbus clouds where vigorous updrafts permit its suspension and growth. When the freezing level is far enough above the surface, most precipitation particles melt and thus form raindrops before they reach the surface, with the exception of large hailstones. Denver Post

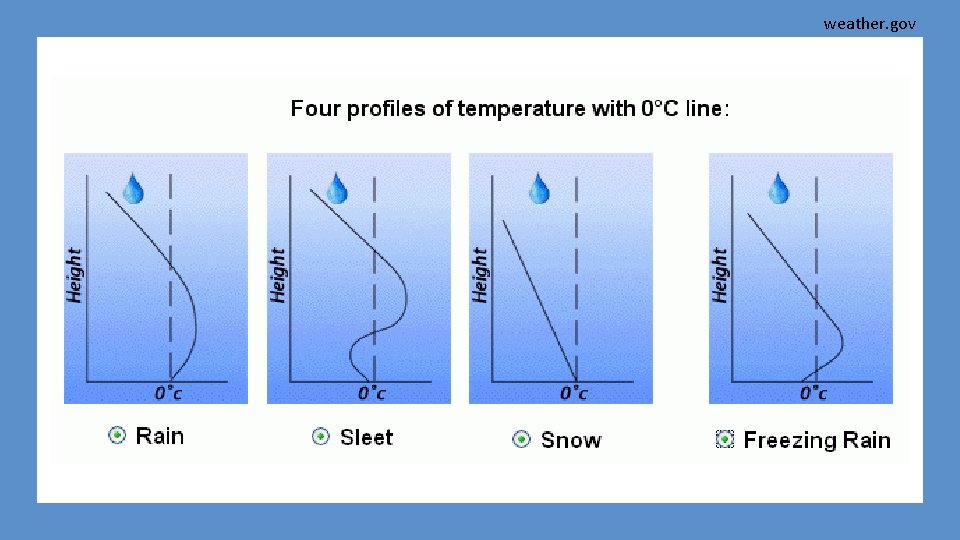
weather. gov