Chronic diseases 1 Chronic diseases have long and


















- Slides: 24

Chronic diseases 1. Chronic diseases have long and variable preclinical phases. 2. The preclinical phase is that portion of the disease natural history during which the disease is potentially detectable, but unrecognized.

Chronic diseases 3. One can conceive of complex functional relationships (sensitivity function) between time in preclinical phase and the sensitivity of a screening test for disease.


Chronic diseases 4. Lead time is the interval between the time of disease detection through screening and the time of disease recognition in the absence of screening. The lead time produced by a screening program for a given individual depends on the time of screening, in relation to the preclinical phase, and on the sensitivity function of the screening test.

Chronic diseases 5. The relative success of treatment for screen detected disease depends on the earliness of detection (or the amount of lead time produced by screening).


Chronic diseases 6. Some chronic diseases have relatively short preclinical phases (on average), without much variability between diseased individuals. Some chronic diseases have very long preclinical phases (on average), with sizable variability between diseased individuals.

Chronic diseases 7. The distribution of lead times produced by a screening program depends on durations of the preclinical phase, on the periodicity (or frequency) of screening, and on the sensitivity function of the screening test.

Chronic diseases 8. Pseudodisease is a condition known only as a result of screening, a condition that would have remained unrecognized if not for screening. Pseudodisease is an instance of screendetected disease with indefinitely long lead time. Pseudodisease includes screen-detected cases which would have regressed (even in the absence of treatment), screen-detected cases which would not have pro-gressed (even in the absence of treatment), and screendetected cases with very long lead times, relative to remaining life expectancy.

Chronic diseases 9. The success of secondary prevention depends on progressive disease existing in a preclinical phase, a screening test capable of detecting disease in the preclinical phase, and the production of lead times (coupled with treatments) sufficient to alter long term outcomes from disease.

Difficulties associated with inferences based on prognosis 1. Prognosis refers to outcomes among persons known to have disease, whether screen-detected or symptom-detected. Case-fatality is a measure of prognosis.

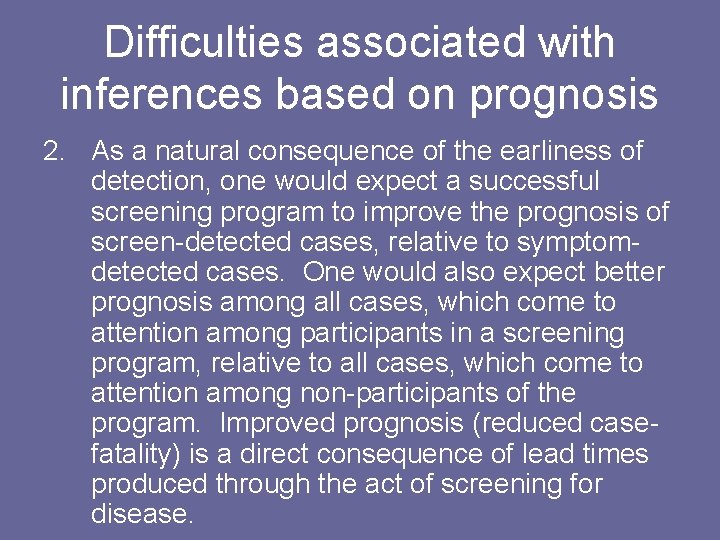
Difficulties associated with inferences based on prognosis 2. As a natural consequence of the earliness of detection, one would expect a successful screening program to improve the prognosis of screen-detected cases, relative to symptomdetected cases. One would also expect better prognosis among all cases, which come to attention among participants in a screening program, relative to all cases, which come to attention among non-participants of the program. Improved prognosis (reduced casefatality) is a direct consequence of lead times produced through the act of screening for disease.

Difficulties associated with inferences based on prognosis 3. However, two important biases influence prognosis among screen-detected cases, even in the absence of any benefit from treatment.

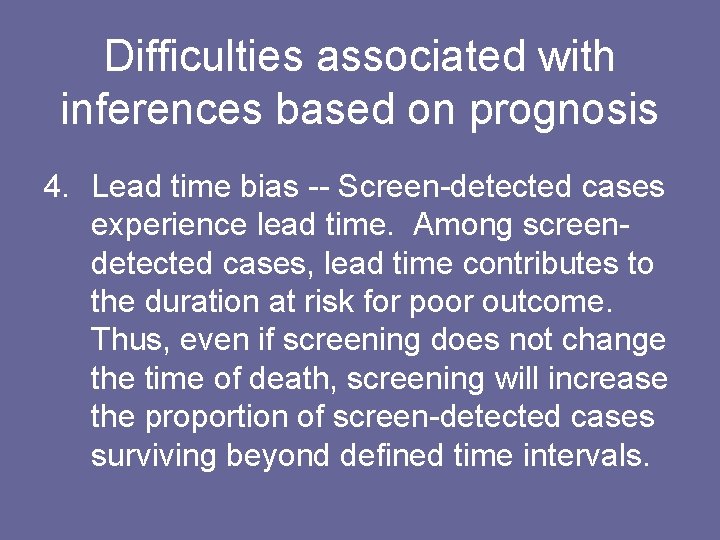
Difficulties associated with inferences based on prognosis 4. Lead time bias -- Screen-detected cases experience lead time. Among screendetected cases, lead time contributes to the duration at risk for poor outcome. Thus, even if screening does not change the time of death, screening will increase the proportion of screen-detected cases surviving beyond defined time intervals.

Difficulties associated with inferences based on prognosis 5. Length biased sampling -- Intermittent screening preferentially detects cases with a long preclinical phase; that is, cases of less rapidly progressive disease. These persons would be expected to experience a relatively favorable outcome, even if allowed to progress to symptoms.

Difficulties associated with inferences based on prognosis 6. The effects of lead time bias and length biased sampling are difficult to separate from the effects of treatment. Therefore, prospective evaluations of the efficacy of screening must compare outcomes among all persons exposed to screening with outcomes among all persons not exposed to screening.

Prospective evaluation

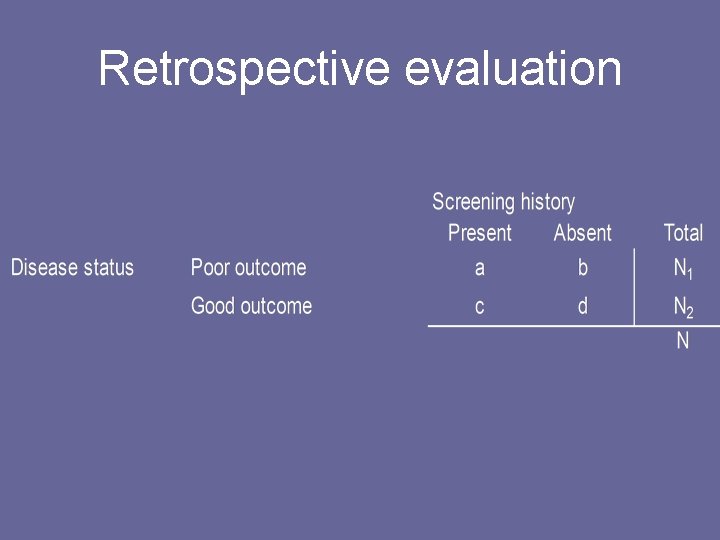
Difficulties associated with inferences based on prognosis 7. Retrospective evaluations of the efficacy of screening must compare cases who experienced a preventable adverse disease outcome and controls who are at -risk for the adverse disease outcome. Remote history of screening during the preclinical phase constitutes the relevant exposure.

Retrospective evaluation

Effects of periodic screening 1. Upon initiation of a new screening program, the prevalence of preclinical disease in the population depends on the incidence of preclinical disease and on the average duration of preclinical disease. 2. The first round of screening preferentially removes prevalent cases of disease with relatively little time remaining in their preclinical phase.

Effects of periodic screening 3. Subsequent rounds of screening have the potential of detecting new individuals (incident cases) entering their preclinical phase since the last round of screening. 4. Depending on the periodicity of screening, in relation to the average duration of the preclinical phase, cases detected during subsequent rounds of screening include a high proportion of incident cases with relatively long lead times. Relative to cases detected at the first screening encounter, cases detected during later encounters may experience more benefit (on average) from early treatment of disease.

Estimating sensitivity in the absence of a gold standard 1. Sensitivity is the proportion detected by a screening test among all cases in the preclinical phase. (Sensitivity is the area under the sensitivity function, weighted according to the distribution of times remaining in the preclinical phase). 2. The sensitivity of a screening test will depend on any factor which changes the distribution of times remaining in the preclinical phase (such as, age of screened group, previous screening history, self-referral status, level of awareness of disease in the population).

Estimating sensitivity in the absence of a gold standard 3. The calculation of sensitivity requires an ability to distinguish false negatives from true negatives. In practice, the application of the gold standard may require invasive or expensive diagnostic testing. It may be inappropriate or unacceptable to perform such testing among persons with negative screening test results. Moreover, even the so-called "gold standard" may fail among cases early in the preclinical phase. But, screening programs attempt to detect disease as early as possible in the preclinical phase.

Estimating sensitivity in the absence of a gold standard 4. Indirect calculation of sensitivity (detection method) = ratio between screen-detected cases and screen-detected plus interval cases. This calculation may overestimate sensitivity. 5. Indirect calculation of sensitivity (incidence method) = 1 - ratio of interval disease incidence rate in a screened group and the disease incidence rate in a comparison group. May underestimate sensitivity.
 Prescriptive screening vs prospective
Prescriptive screening vs prospective Global alliance against chronic respiratory diseases
Global alliance against chronic respiratory diseases Tall+short h
Tall+short h The gingerbread man once upon a time
The gingerbread man once upon a time It has six faces 12 edges 8 vertices
It has six faces 12 edges 8 vertices Tinikling costume and props
Tinikling costume and props Long long ago when the gods and goddesses
Long long ago when the gods and goddesses Long long ago people used to think that the earth was
Long long ago people used to think that the earth was Once upon a time there was a great king
Once upon a time there was a great king Once upon a time long long ago
Once upon a time long long ago Long long int c
Long long int c Lông vằn lông vện mắt xanh
Lông vằn lông vện mắt xanh Once upon a long time ago
Once upon a long time ago Lông vằn lông vện mắt xanh
Lông vằn lông vện mắt xanh Lifestyle modern
Lifestyle modern Communicable and noncommunicable diseases venn diagram
Communicable and noncommunicable diseases venn diagram Section 19-3 diseases caused by bacteria and viruses
Section 19-3 diseases caused by bacteria and viruses Define a primary skin lesion and list three types
Define a primary skin lesion and list three types Chapter 6 musculoskeletal system diseases and disorders
Chapter 6 musculoskeletal system diseases and disorders Chapter 24 sexually transmitted diseases and hiv/aids
Chapter 24 sexually transmitted diseases and hiv/aids Chapter 22 genetics and genetically linked diseases
Chapter 22 genetics and genetically linked diseases Chapter 21 mental health diseases and disorders
Chapter 21 mental health diseases and disorders Chapter 17 reproductive system diseases and disorders
Chapter 17 reproductive system diseases and disorders Chapter 15 nervous system diseases and disorders
Chapter 15 nervous system diseases and disorders Nail diseases and disorders milady
Nail diseases and disorders milady