Chemistry Properties of Matter Chemistry Pretest Work Samples









- Slides: 15

Chemistry: Properties of Matter

Chemistry Pretest!!!

Work Samples

New Sticky Tab The Chemistry unit begins on page 26. Chemistry Picture Word Choices are… matter molecule chemistry transformation* rearrangement* photosynthesis* solid. liquid. gas* temperature* * Challenge words

Page 27: Tuesday, 4/2/13 Today’s Agenda Properties of Matter Today’s Learning Target I can define and explain 4 properties of matter.

Page 27 Notes: Matter 1. Matter is the material that makes up everything around us. 2. All matter is made up of microscopic bits like atoms and molecules. 3. Some properties of matter include: -State -Color -Inertia -Boiling Point -Solubility -Composition -Mass -Volume -Density

Table Talk • What are two things made of matter? • What are two things NOT made of matter? How do you know these are not matter?

Page 27 Notes: STATE 4. Matter can be in a solid state, liquid state, or gaseous state. 5. Solid matter is made of molecules that are moving very slowly. Solids are fairly hard, stable and hold their own shape regardless of the shape of their container. 6. Liquid matter is made of molecules that are moving more quickly. Liquids are wet and fluid substances which settle to fill the bottom of their container. 7. Gases are matter made of molecules that move about very quickly. Gases are usually invisible, can escape their containers and can be inhaled.

Page 28 Notes: BOILING POINT 8. The boiling point of a substance is the temperature at which it changes from a liquid to a gas. -different substances boil at different temperatures. -water boils at a temperature of 100°C. -gold boils at 2, 856°C

Target Describe 4 properties of matter.

TARGET 1. Reread today’s learning target. 2. Think about what you learned today. 3. Write a letter grade: A, B, C, D or F for yourself next to the target. (A+, A-, …) 4. Be honest. This grade is meant to help you know your strengths and weaknesses. 5. Be prepared to show well you can do or understand today’s target.

Page 28 Notes: SOLUBILITY 9. Solubility is a substance’s ability to be dissolved in liquid. -Some materials, like salt or sugar disappear easily into a glass of water.

Page 28 Notes: MASS 10. When you use a balance to find the mass of an object, you are really measuring how much matter it has. - Unlike weight, Your mass will remain the same regardless of gravity’s pull.

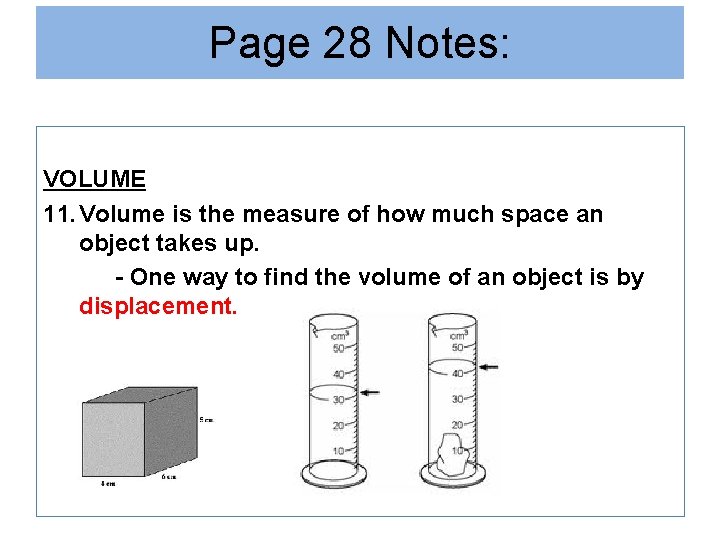
Page 28 Notes: VOLUME 11. Volume is the measure of how much space an object takes up. - One way to find the volume of an object is by displacement.

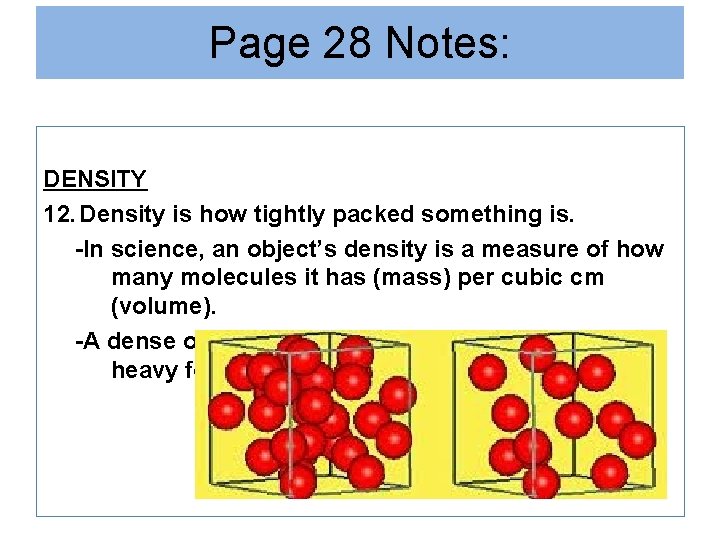
Page 28 Notes: DENSITY 12. Density is how tightly packed something is. -In science, an object’s density is a measure of how many molecules it has (mass) per cubic cm (volume). -A dense object can be recognized because it is heavy for its size.
 Introduction for portfolio in math
Introduction for portfolio in math Section 1 composition of matter
Section 1 composition of matter Gray matter in the brain
Gray matter in the brain Section 1 composition of matter
Section 1 composition of matter Chapter 2 section 1 classifying matter answer key
Chapter 2 section 1 classifying matter answer key Label the cranial dura septa and associated sinuses.
Label the cranial dura septa and associated sinuses. Composition of matter section 1
Composition of matter section 1 Gray matter and white matter
Gray matter and white matter Pallium telencephalon
Pallium telencephalon Ecological succession
Ecological succession Pretest: developing an academic and career plan
Pretest: developing an academic and career plan Pretest: the early and mid-nineteenth century: romanticism
Pretest: the early and mid-nineteenth century: romanticism Medication administration 1 pretest
Medication administration 1 pretest Intravenous medication administration pretest
Intravenous medication administration pretest Pretest: growth, development, and sexuality
Pretest: growth, development, and sexuality Pre test publicitario
Pre test publicitario